Key Points
■ Imaging studies have limited value in the differentiation between viral and bacterial lower respiratory tract infections (moderate evidence).
■ CT provides more information than plain radiographs for complicated pulmonary infections with empyema, pleural effusion, or bronchopleural fistula (moderate evidence).
■ In immunocompromised patients, CT has been shown to characterize the type of infection better than plain radiographs (moderate evidence).
■ Ultrasound has an advantage over CT in the identification and characterization of complicated effusions (moderate evidence).
■ Early detection and therefore intervention for pleural complications of pneumonia are critical and can result in better outcomes (moderate evidence).
■ Early surgery (VATS) is more cost-effective than thoracotomy (without or with image guidance) in the treatment of empyemas in children (strong evidence).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Definition and Pathophysiology
Pneumonia is defined as an infection of the lungs. Acute respiratory infections are the most common infection of the human host. The majority of these are of the upper respiratory tract, but infections of the lower respiratory tract are frequent challenges for clinicians caring for children. Most of these illnesses are mild, and patients suffering from them are appropriately cared for in an ambulatory setting, but a small number are ill enough to require hospitalization and some die. Thus, lower respiratory tract infections, particularly pneumonia, constitute a major health problem both in the United States and throughout the world. Bacterial pneumonias make up only a small number of lower respiratory tract infections but have the highest mortality rates. The infectious agents responsible for pneumonia and lower respiratory tract infection can be divided into bacterial, viral, fungal, mycobacterial, and atypical causes. Incidence of specific pathogens differs by age (Table 27.1). The most common pathogens in children are Streptococcus pneumoniae and Haemophilus influenzae, followed by Staphylococcus aureus and Streptococcus pyogenes (1–5). In particular, S. aureus and gram-negative pathogens can affect newborns and malnourished children (5). Viral illnesses such as respiratory syncytial virus (RSV), influenza, parainfluenza, and adenovirus have been identified in approximately a quarter of children with pneumonia (5). The potential complications of pneumonia include parapneumonic (pleural) effusion, empyema, or abscess. Empyema is treated either medically, surgically, or with placement of drainage tubes. Abscesses and necrotizing pneumonias are usually treated medically, but interventional procedures such as drainage tube placement might sometimes be used.
Pleural Effusion and Empyema
A pleural effusion is broadly defined as an abnormal collection of fluid in the space between the parietal and visceral pleuras and may arise from a variety of processes that alter the normal flow and absorption of pleural fluid. The most common causes of pleural effusions in children are pneumonia (parapneumonic effusion), congenital heart disease, and malignancy. A diagnostic or therapeutic thoracentesis is usually indicated to determine the nature of the effusion as well as to relieve associated dyspnea and respiratory compromise.
An empyema is characterized by the presence of pus, with polymorph nuclear leukocytes and fibrin, in the pleural space. Nearly one-half of all children with pneumonia will develop parapneumonic effusions, but fewer than 5% of these effusions progress to empyema. However, the incidence of empyema complicating community-acquired pneumonia is increasing, which causes significant childhood morbidity (6). Progression of a pleural effusion to empyema is through a three-stage evolution: (1) exudative, (2) fibrinopurulent, and (3) organization. Most commonly pleural effusions do not progress beyond the exudative phase and resolve with antibiotics alone (4, 7). The second stage is heralded by the arrival of bacteria, by pleural invasion from the contiguous pneumonic process. Progression occurs with polymorph accumulation and fibrin deposition; membrane formation occurs and the developing empyema may become compartmentalized or loculated. The chemistry profile of pleural fluid is characterized by a decrease in pH and glucose concentrations and an increase in lactate dehydrogenase concentration (LDH). The most common organisms encountered in pleural fluid in children are S. aureus, S. pneumoniae, H. influenzae, and S. pyogenes (1–5). If a fibropurulent pleural effusion is not adequately treated, then the third organizing phase develops. Fibroblasts grow into the exudate from the visceral and parietal pleural surfaces. An inelastic membrane is formed, which and may encase the lung and pleura, with the potential to restrict respiration. The thick exudate may drain spontaneously through the chest wall or into the lungs, causing a bronchopleural fistula. Other infectious complications of empyema include bacteremia and pericarditis by direct extension or bacteremic seeding, and pneumothorax.
Lung Abscess
A lung abscess is an accumulation of inflammatory cells accompanied by tissue destruction or necrosis that produces one or more large cavities in the lung. It is arbitrary to designate larger cavities by the term lung abscesses and smaller, multiple cavities with similar histology by the term necrotizing pneumonia. Necrotizing pneumonia is an acute fulminating infection of the lung parenchyma, characterized by vascular thrombosis and rapid tissue breakdown leading to multiple thin-walled cavities. Pulmonary abscess is a more chronic thick-walled cavity in the lung, usually encountered in patients with chronic debilitating states. Anaerobic bacteria and S. aureus are most frequently implicated with the formation of a lung abscess. Complications of lung abscess include rupture into adjacent compartments, which occurs more frequently with S. aureus. Localized bronchiectasis may also occur as a complication.
Epidemiology
According to the WHO, there are over 150 million cases of pneumonia annually in the world in children less than 5 years of age (8). In the United States, 20 million hospitalizations in the pediatric population occur, although many are of viral etiology (8). Each year in the developed nations, 5–10% of children under the age of 5 years will develop pneumonia (5). The incidence of pneumonia in children is approximately 34–40 per 1,000 (9). Approximately one-half of children younger than 5 years with community-acquired pneumonia require hospitalization (10). Complicated pneumonia such as necrotizing pneumonia and abscess formation has been noted to increase in incidence over the years between 1990 and 2005 (11, 12). Parapneumonic effusions complicate pneumonia between 36 and 56% of cases with an incidence between 0.4 and 0.6 per 1,000 cases (13). Empyema complicates an estimated 0.6% of all childhood pneumonias, resulting in an incidence of 3.3 per 100,000 children (6). Unlike in the adult population, most pediatric patients recover without mortality whereas in adults, the mortality rate has been reported to be up to 20% (6, 11). There has been an emergence of community-acquired methicillin-resistant S. aureus (MRSA) in pediatric pneumonia, originally thought to be mainly hospital acquired (14). An aggressive infection, MRSA pneumonia, has been reported to present with high fever, leukopenia, rapidly progressing respiratory distress, development of multilobar infiltrates with effusions, and empyema (15, 16). Intravenous antibiotics are first-line therapy and surgical treatment may be needed in patients with MRSA pneumonia complicated by empyema (15).
Overall Cost to Society
In the adult population in Europe, the cost to society resulting from uncomplicated pneumonia is estimated at $8 billion, with 1.1 million cases per year (17). Childhood pneumonias are a frequent cause of doctor visits, antibiotics prescriptions, loss of work days of parents, and reduction of quality of life (18). For example, the mean number of work days lost by mothers ranged between 0.2 and 4.2 days (18). No additional specific US pediatric cost data were found in the literature. However, there has been one cost-effectiveness analysis reported, related to pneumonia with pleural involvement, treated by either thoracentesis, chest tube/pleural drain placement, or video-assisted thoracoscopic surgery (VATS) (13).
Length of hospitalization has been compared between different treatment strategies of parapneumonic effusions, or empyema. In a randomized controlled trial, gross total cost of hospitalized patients requiring intervention to treat parapneumonic effusions has been demonstrated to be approximately equal for treatment with VATS versus chest tube placement, resulting in total charges of approximately $21,947 versus $19,714 on average, respectively. However, hospital length of stay was on average 6 days for patients who underwent VATS and 13 days for patients who underwent chest tube placement (13).
Goals
The main goal in imaging pulmonary infections is early diagnosis. This will aid in early and adequate treatment and may prevent potential complications. When there is pleural effusion, imaging guides appropriate management. The standard treatment of pneumonia and its complications may include all or part of the following: antibiotics, thoracentesis, chest tube placement, fibrinolytic therapy, or surgical debridement of empyema. Severe cases of empyema, for example, often require surgical decortication if there is failure to respond to antibiotic treatment, thoracentesis, or chest tube placement. Appropriate diagnosis and treatment must aim to minimize risk to patients, including that related to ionizing radiation exposure, interventional procedure complications, and surgical complications.
Methodology
The review of the current diagnostic imaging literature was done using PubMed/MEDLINE covering the years January 1980–December 2008. The search strategy used the following key statements and words: pediatric, children, neonate, neonatal, pneumonia, empyema, pleural effusion, abscess, VATS. We excluded non-English journal articles, case reports, animal studies, and basic science articles.
Discussion of Issues
I. What Are the Clinical Presentation and Predictors of Chest Infections in Children, and Which Findings Raise the Suspicion for Complications?
Summary of Evidence: Clinical presentation and diagnosis of pneumonia vary with age and pathogen, with tachypnea being the single most predictive sign (moderate evidence).
The clinical exam does not reliably diagnose pneumonia nor distinguish between viral and bacterial pneumonias (moderate evidence).
Supporting Evidence: The goal of clinical evaluation is to confirm the clinical diagnosis and to assess severity of disease (10). The typical presentation is cough and respiratory distress, sometimes with fever and muscle aches. History, physical examination, laboratory testing, and radiographic testing are the primary components of the diagnostic workup. History focuses on assessment of age, presence of fever, chest pain, dyspnea, immunizations, duration of symptoms, exposure to sick contacts, and recent travel. In a study of 110 children with respiratory infections, tachypnea emerged as the best clinical sign for identifying pneumonia, with a sensitivity of 74% and a specificity of 67% (19). Although there may be findings suggestive of either viral or bacterial pneumonia, the clinical exam does not reliably diagnose pneumonia nor distinguish between viral and bacterial pneumonias. Models that have been tested in adult populations based only on clinical information in the absence of imaging do not reliably predict the presence of pneumonia (20).
Laboratory evaluation includes a complete blood count and leukocyte differentiation. In children with severe symptoms or if outbreak is suspected, microbiology of sputum and blood cultures may be useful (21, 22). The diagnosis of pneumonia is often confirmed and defined by the presence of a lung opacity on chest radiography. Accepted indications for a chest radiograph include severe disease, confirmation of non-specific clinical findings, assessment of complications, and exclusion of other thoracic causes of respiratory symptoms (23, 24).
In the neonatal period, pneumonia is virtually always due to bacteria, particularly group B Streptococcus as well as E. coli, H. influenzae, and –Listeria monocytogenes (25). Physical findings and clinical signs of pneumonia are often non-specific but include fever, temperature instability, difficulty feeding, and restlessness (10). In this population, viral pneumonia is rare due to conferred maternal antibody protection, whereas bacterial pneumonia is most frequently due to pathogens acquired during labor and delivery and is more prevalent in premature babies. In keeping with dropping maternal antibody levels, viral childhood pneumonia occurs at a peak between 2 months and 2 years of age (7). Presenting signs and symptoms in this age group remain non-specific and include cough, fever, temperature instability, abnormal leukocyte count, findings of sepsis, and respiratory distress (10). In older children from 2 years to 18 years of age, bacterial infections become relatively more common. In addition to previously mentioned symptoms, pleuritic chest pain may occur (7, 10). In a retrospective case series of 79 patients who presented with at least one symptom of fever, cough, sputum production, chest pain, dyspnea, or course crackles, pneumonia was diagnosed using chest radiography as the gold standard in 24 (prevelance: 30%) (26). In this study group, a combination of all four symptoms of fever, cough, sputum, and course crackles yielded a sensitivity and specificity of 91.7 and 92.7% for clinical detection of pneumonia.
Viral Versus Bacterial Pneumonia
If possible, differentiation between bacterial and viral etiologies of pneumonia would be helpful for treatment decisions. As mentioned previously, age is an important criterion. In bacterial infection, pulmonary findings are most commonly limited to one anatomic area on physical examination, and symptoms include fever, moderate-to-severe respiratory distress, and chest pain (27). Primary viral infection is considered more likely if symptoms include wheezing (27). There is a gradual onset of symptoms such as fever, congestion, rhinorrhea, and wheezing (27–29). Children with viral pneumonias also tend not to appear as toxic as those with bacterial pneumonia (28). In addition, viral illnesses may often precede bacterial infection (27).
Lung response to an infective antigen may be more age specific than antigen (i.e., bacteria versus viral) dependent. Virkki et al. performed a study in 254 children admitted with diagnosis of community-acquired pneumonia to evaluate the role of chest radiography, total white blood cell count, serum C-reactive protein, and erythrocyte sedimentation rate (30). They found that 71% of children with lobar opacities demonstrate laboratory evidence of a bacterial infection, but interstitial opacities were seen with approximately equal frequencies in viral and bacterial pneumonias. Of children less than 2 years, 38% had bacterial infections and 60% viral, whereas in older children bacterial pneumonias were more prevalent (49%) than viral (22%), the remainder being of mixed etiology. Likewise, Korppi et al. performed a study of 61 children treated for radiologically and microbiologically confirmed viral or bacterial pneumonia (31). Chest radiographs were independently reviewed by two radiologists, and they found that 74% of the patients with alveolar and 62% with interstitial pneumonias had bacterial infection. An interstitial pattern of pneumonia on chest radiographs is therefore non-specific: it could be due to viral (26%), bacterial (30%), or mixed (44%) etiologies. Hence, distinguishing between viral and bacterial pneumonias in pediatric patients remains problematic, given the number of clinical and radiographic findings that overlap.
Parapneumonic effusions and empyema in children are complications and follow acute bacterial pneumonias (12, 32). Rare causes of empyema include distant spread from other sources of infection such as osteomyelitis of the ribs, septic emboli, and lung abscesses. Children typically present ill-appearing, febrile, and with unilateral chest signs (12, 32).
II. When Are Chest Radiographs Useful in Children with Suspected Pneumonia?
Summary of Evidence: Chest radiographs may have a role in evaluating for pneumonia in the clinical presentation of fever of unknown origin (limited evidence).
Chest radiographs are sufficiently sensitive and highly specific for the diagnosis of community-acquired pneumonia (moderate evidence).
Imaging studies have limited value in the differentiation between viral and bacterial lower respiratory tract infections (moderate evidence).
Supporting Evidence
Fever of Unknown Origin
There are currently varying data regarding the utility of chest radiographs for fever of unknown origin. Only 0–3% of infants with fever of unknown origin had a positive chest radiograph demonstrating pneumonia, and therefore chest radiography appears to have limited utility in this age group (33, 34). This contrasts with a prospective study of 278 children aged less than 5 years with fever and leukocytosis, to determine the incidence of radiographic findings of pneumonia, which was found not only in 40% of those with clinical findings suggestive of pneumonia but also in 26% of those without clinical evidence of pneumonia. Accounting for the 53 children in whom no chest radiograph was taken and who were presumed to not have pneumonia, this study estimated the minimum incidence of clinically occult pneumonia in this population as 19% (35).
Neonatal Pneumonia
Chest radiographs are commonly used in the neonatal intensive care unit, but the radiographic findings of neonatal pneumonia substantially overlap with those of other causes of the neonatal respiratory distress syndrome. Although evidence for this practice is lacking, a negative chest radiograph result allows the neonatologists to stop antibiotic treatment at 3 days of age, if other tests are also negative.
Community-Acquired Pneumonia
Chest radiography is the standard first-line imaging tool for the evaluation of suspected pulmonary infections, particularly in suspected community-acquired pneumonias. However, a Cochrane review of a randomized trial of 522 children, aged 2 months to 5 years, performed by Swingler and Zwarenstein failed to demonstrate any evidence that chest radiography improves outcome in ambulatory children with lower respiratory tract infection (36). Up to 10% of pediatric patients with proven pulmonary infection can have a normal chest radiograph (sensitivity 90%) (17). Table 27.2 summarizes the test characteristics of the only three studies in which complete sensitivity and specificity data of chest radiography are available: reported sensitivities range between 71 and 87% and specificities from 90 to 98% (37–39). In a few more limited studies, sensitivity and specificity values were not directly specified, but accuracy was reported to range between 58 and 77% (38–40).
Chest Radiography
In the evaluation of children with pneumonia, frontal views are most useful when lobar (bacterial) pneumonia is present. When pneumonia has been defined as “a focus of streaky or confluent lung opacity,” the sensitivity and specificity of the frontal view alone were 85 and 98%, respectively. For opacities that are confluent and lobar in distribution (not streaky or non-segmental/non-lobar), the sensitivity and specificity increased to 100% (37). However, this study also suggested that non-lobar types of infiltrates will be underdiagnosed in 15% of patients if only the frontal view is used.
Differentiation of Bacterial and Viral Pneumonias
When viral causes of respiratory infection such as bronchiolitis are suspected, chest radiographs may not be needed in uncomplicated cases. In a retrospective study of 298 patients in an urban children’s hospital at the University of Colorado by Roback et al., clinicians did not typically obtain chest radiographs in first-time wheezing episodes, whereas there was a higher utilization of radiographs in patients with elevated temperature, absence of family history of asthma, and localized wheezing on physical examination (40). Perlstein et al. developed a publication of a set of evidence-based guidelines, as implemented at the Children’s Hospital of Cincinnati, that demonstrated 20% decrease in the number of chest radiographs ordered (40, 41). In another study of 72 adult patients by Graffelman et al. in the primary care setting, limited value was found using chest radiography in predicting the etiology of viral versus bacterial lower respiratory infections. The positive predictive value and the negative predictive value for bacterial infection were 75 and 57%, respectively (42).
III. How Does Chest Radiography Compare to Cross-Sectional Imaging in the Evaluation of Chest Infections in Children? When Is Chest CT Indicated?
Summary of Evidence: Chest CT is not warranted in uncomplicated pneumonia (moderate evidence).
CT provides more information than plain radiographs for complicated pulmonary infections with empyema, pleural effusion, or bronchopleural fistula (moderate evidence).
In immunocompromised patients, CT has been shown to characterize the type of infection better than plain radiographs (moderate evidence).
Supporting Evidence: Given satisfactory performance of chest radiographs in uncomplicated community-acquired pneumonias, CT is not recommended for evaluation of pulmonary infections without empyema, pleural effusion, or bronchopleural fistula.
Pneumonia with Complications (Table 27.3 )
Several studies (43–50) have demonstrated that CT can often add information to the diagnosis, particularly in fungal infections and in complicated pneumonia cases. In a case series of 42 immunocompetent children, chest radiography was suboptimal in detecting abscesses, bronchopleural fistulae, fluid loculations, and parenchymal involvement when compared to CT (51). Chest radiograph accuracy rates were reported as follows: fluid loculations (42%), abscess formation (40%), bronchopleural fistulae (33%), and parenchymal involvement (84%). A limitation of this study is the lack of reported sensitivity and specificity values. Donelly and Klosterman performed a study of 56 patients with complicated pneumonia who were not responding to treatment. Chest CT was compared to a chest radiograph performed earlier on the same day. CT scans were evaluated for the presence of cavitary necrosis, abscess, bronchopleural fistula, cavitation, loculated pleural effusions, malpositioned chest tube, pericardial effusion, or bronchial obstruction (50). All 56 CT scans demonstrated at least one of the above findings that were not seen on chest radiographs (50). A total of 110 findings were seen on CT and not on chest radiography, with an average of approximately two findings per CT scan. Parenchymal complications totaled 40 and pleural complications 37. In another retrospective analysis of 17 children who underwent both CT scanning and chest radiography, evidence of cavity necrosis is often seen on CT before or in the absence of findings on chest radiographiy (52).
Immunocompromised Children with Pneumonia
In the high-risk immunocompromised patients, it is absolutely critical to have a high sensitivity, as failure to detect results in failure to treat and subsequent high mortality (45). CT has been shown to have higher accuracy than plain radiography for early detection of pneumonia in immunocompromised and hospitalized patients (45, 48). For example, in a series of 48 patients (median age of 11 years and range of 2–19 years), chest radiographs and CT were rated independently by three experienced radiologists and subsequently correlated with biopsy or bronchoscopic washing results. CT was shown to identify more true-positive cases of bacterial and fungal pneumonias than radiography (91 versus 85%). Unfortunately, no detailed numbers of sensitivity and specificity were cited (45). In 87 adult patients with febrile neutropenia (median age 47, range 18–80 years), CT detected pneumonia 5 days on average earlier than chest radiographs and was more sensitive in the detection of poorly defined opacities, ill-defined nodules, consolidation, ground-glass opacities, pleural effusions, cavitations, and bullae (53). For the evaluation of children who are severely ill or immunocompromised for fungal infections and Pneumocystis carinii pneumonia (PCP), CT can add value. Janzen et al., in a retrospective review of 45 children who underwent both CT and chest radiography, found that the first-choice diagnosis was correct in 44% on chest CT and correct in 30% on chest radiography (p < 0.05) (49). Equivocal or normal chest radiographs are common, reported up to 39% in patients with PCP infection and up to 10% of patients with known pulmonary disease (17). CT can aid in the detection of fungal infections via identification of nodules, cavitation, ground-glass opacities, and halo effect (45–47). CT can play an important role such as in evaluating pulmonary aspergillosis and candidal pneumonias (45). In another study to evaluate if CT adds information to chest radiography, 33 cases were reviewed retrospectively (54). It was found that in 16 cases CT added no additional useful information, but in 17 cases CT added confidence and changed management (biopsy, changing antibiotics, bronchoscopy).
Nosocomial Infections and MRSA Pneumonia
In the case of nosocomial infections, such as multidrug-resistant infections (MRSA pneumonia), CT offers a rapid and decisive diagnosis (14). For aspiration pneumonias, chest radiographs alone are usually adequate for diagnosis and to ensure resolution. The primary role of imaging has been the detection and monitoring of complications from the pneumonia.
IV. What Is the Role and Diagnostic Performance of Imaging Studies (Radiography, Ultrasound, and CT) for Treatment Planning of Complicated Pneumonia with Empyema and Parapneumonic Effusions?
Summary of Evidence: Chest imaging can be used to distinguish between the exudative and organizing phases of pleural effusion, through demonstration of atypical layering patterns on decubitus radiography, complexity of pleural fluid on ultrasound, and loculations of heterogeneous fluid on CT (moderate evidence).
Ultrasound has an advantage over CT in the identification and characterization of complicated effusions, also being more cost-effective and not employing ionizing radiation (moderate evidence).
Supporting Evidence: The diagnosis of pleural effusion can be readily achieved via radiography, ultrasound, or CT. There are several studies evaluating the prognostic implications of the use of ultrasound versus CT and the implications for treatment decisions. Ultrasound can be helpful in both prognosis and treatment decisions. It is a low-cost test, widely available, portable, does not use ionizing radiation, and rarely requires sedation. This has to be contrasted to CT, which has a relatively high radiation dose, in the order of 100 times that of a chest radiograph. Ultrasound is effective in demonstrating “high-grade” effusions containing septations, fronds, loculations, and debris. US depiction of the thickness and number of these septations predicts the success of chest tube drainage (55). CT assists in providing a global assessment for pre-therapy planning, including the ability to demonstrate size, possible loculations, and the extent of lung involvement, including abscess formation or necrosis (51). Kearney et al. demonstrated in a retrospective review of 50 patients who underwent both US and CT that, although both US and CT have effective roles, neither technique reliably identified the stage of pleural effusions or predicted whether patients would require surgical intervention (56).
Donnelly et al. have shown in a retrospective review of 30 patients who received a chest CT with subsequent pleural fluid analysis that CT characteristics of a parapneumonic effusion do not allow one to predict empyema and distinguish it from a transudative process (57, 58). Experienced radiologist reviewers rated the presence of pleural enhancement (seen in 100% of empyemas and in 89% of transudative effusions), pleural thickening (57 and 56%, respectively), abnormal extrapleural space (66 and 67%, respectively), and extracostal chest wall edema (33 and 56%, respectively) (57).
A retrospective analysis of 46 pediatric patients with empyema by Ramnath et al. found that early sonographic evaluation of parapneumonic effusions led to decreased hospital length of stay for high-grade effusions and effective triage into two groups, operative (20 patients) versus non-operative treatment (26 patients) (55). Operative therapy included video thoracotomy, open decortication, or pleural debridement, and non-operative therapy included antibiotics alone with or without thoracentesis or chest tube placement. When the ultrasound detected high-grade effusions (evidence of organization such as fronds, septations, or loculations), the hospitalization stay was shorter (8.6 days) for patients eventually treated operatively, compared to those who were not (16.4 days). In non-operated patients in whom ultrasound revealed low-grade effusions, there was no significant difference in hospital stay length between patients treated with antibiotics alone and chest tube drainage (55). In another study, ultrasound has also been shown to be quite effective in identifying empyemas (59). In this prospective study of 1640 febrile patients admitted to the ICU, 94 patients underwent 118 ultrasound-guided thoracenteses for pleural effusions, identified by chest radiography and ultrasound. In 11/31 studies where a complex septated pattern and in all four where a diffusely hyperechoic pattern was reported, empyemas were confirmed with thoracentesis. On the other hand, in none of the remaining 83 studies where an anechoic or complex non-septated/hypoechoic pattern was reported were empyemas diagnosed.
V. What Are the Relative Roles of Imaging in Medical Therapy, Minimally Invasive Intervention such as Thoracotomy or Thoracentesis, and Surgical Treatment for Pneumonia Complicated by Pleural Involvement?
Summary of Evidence: Early detection of complicated pneumonia by imaging and subsequently early intervention result in better patient outcomes (moderate evidence).
Currently, there is no strong evidence to support or not support fibrinolytic therapy for childhood empyema in conjunction with image-guided interventions (insufficient evidence). While it may result in more rapid treatment response, side effects and the pain from this treatment limit its current use.
There is strong evidence in the surgical literature that early surgery (VATS) is more cost-effective than thoracotomy (without or with image guidance) in the treatment of empyemas in children (strong evidence).
Supporting Evidence
Image-Guided Thoracentesis and Chest Tube Placement
As pleural effusions evolve, antibiotic therapy alone may not be sufficient. More aggressive intervention is often required. Beyond antibiotics, minimally invasive therapy with image-guided thoracentesis or chest tube drainage may be necessary. Thoracentesis is a standard procedure for the management of pleural effusions in adults. However, in children, thoracentesis is not easily performed as this procedure requires cooperation and frequently sedation. Patients treated with thoracentesis have been reported to require additional interventions due to recurrence (4, 6, 60). For example, Tu et al. found in 94 ICU patients with a pleural effusion suspected to have empyema that additional interventions were often needed; a total of 118 image-guided thoracentesis procedures were performed in this group (59). A prospective study of 67 patients with empyemas found that single chest tube placement versus repeated thoracentesis had no significant difference in outcome, thereby supporting that in children early thoracotomy tube drainage is preferred to thoracentesis, in order to avoid repeat interventions (61). In this study, the patients were prospectively divided into two treatment arms: chest tube placement (32 patients) and ultrasound-guided thoracentesis (35 patients). As assessed by chest radiography, the amount of fluid drained, the number of days that the patient was febrile, and the duration of antibiotic therapy, no statistical differences were found between both treatment arms.
Thoracentesis or single chest tube placement may not be beneficial in the presence of loculations and adhesions by fibrin. The aim of using fibrinolytic therapy in such cases is to improve drainage of pus by lysis of the fibrinous bands.
Fibrinolysis
A recent meta-analysis has shown that, in adults, intrapleural fibrinolytic therapy confers significant benefit in reducing the requirements for surgical intervention for patients in the early studies included in the review, but not in the more recently published studies (62). Separate subgroup analysis of proven loculated/septated effusions suggests a potential overall treatment benefit with fibrinolytics, but these results should be treated with caution. In children, fibrinolytic therapy has been reported in three studies with about 64 cases (63). There were no in-hospital deaths. The aggregate data revealed a failure rate of 9.3% and a complication rate of 12.5%. One of the studies was a 10-center randomized prospective study of urokinase undertaken by the British Paediatric Respiratory Society Empyema Study Group (64). They reported a slight reduction in length of stay in hospital compared to the saline group (7.4 versus 9.5 days). However, a major surgical concern with this therapy is that patients may be more likely to fail rescue VATS, as it has been suggested that urokinase causes intrapleural adhesions to become very adhesive (6).
Beyond Image-Guided Thoracentesis or Thoracotomy: Surgery
In cases not amenable to minimally invasive image-guided procedures such as thoracentesis or simple chest tube placement without or with fibrinolysis, surgical interventions including video-assisted thorascopic surgery (VATS) or thoracotomy can be effective options (13, 63). VATS is a relatively safe and effective treatment for complicated pleural effusion and empyema.
Early intervention with VATS can result in better outcomes (13, 65). Luh et al., using VATS, described an 86.3% satisfactory result in 234 patients with complicated pneumonia with effusions or empyema (65). In a prospective randomized trial, Kurt et al. found that early intervention with VATS was superior to conventional thoracotomy drainage (13). These surgical papers may be biased toward operative in favor of image-guided intervention. However, even a meta-analysis, performed to evaluate primary operative versus non-operative therapy in children with empyema, suggested a more favorable response with early surgical intervention (VATS or thoracotomy) (63). In this meta-analysis, primary operative therapy was associated with lower in hospital mortality rate, re-intervention rate, length of stay, time with tube thoracotomy, and time of medical therapy when compared to medical management.
Take Home Tables and Figures
Tables 27.1, 27.2, and 27.3 show data on infectious pneumonia by age, diagnostic performance of clinical exam and chest radiography in detection of pneumonia in non-immunocompromised patients, and a literature review of CT diagnostic performance in all populations of pediatric patients, respectively.
Figure 27.1 is a diagnostic and therapeutic imaging workup algorithm for pneumonia with pleural effusion in immunocompetent children. Figures 27.2 and 27.3 show the role of ultra-
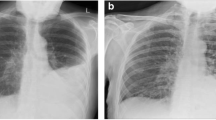
Empyema: role of CT. Radiograph (A) and CT (B) demonstrate left-sided pleural collection with mass effect, consistent with empyema. Radiograph (C) and CJ (D) following chest tube placement show thickening of the visceral pleura consistent with pleural, fibrosis (organization phase, grade 3), preventing full expansion of the left lung.
sound and CT in empyema. Figure 27.4 shows how CT can be used to differentiate empyema from lung abscess.
Use of CT to differentiate empyema from lung abscess. Radiograph (A) and CT (B) demonstrate a fluid collection with mass effect on the lung (arrows), which forms an obtuse angle with the pleura, consistent with a pleural abscess (empyema). Radiograph (C) and CT (D) demonstrate a gas- and fluid-containing lung mass, which forms a sharp angle with the pleura, confirming its intraparenchymal location.
Case Imaging Studies
Case 1
Figure 27.5 presents CT in a child with acute lymphatic leukemia with ARDS.
Suggested Imaging Protocols
Radiography
Posterior–anterior (PA) and lateral views are optimal whenever possible. Anterior–posterior (AP) views are also very useful. In suspected effusions, decubitus views can be useful in distinguishing free-flowing pleural fluid versus loculated fluid collections. However, in the presence of extensive pulmonary parenchymal consolidation, the value of decubitus films to identify loculated versus free pleural fluid is severely limited.
Ultrasound
Technique includes screening of the whole pleural space, not just the lung bases. Lower frequency (3.5–7 MHz) sector transducers are used initially for more overview through inter- and subcostal scanning; higher frequency (10–12.5 MHz) linear transducers are helpful for more detail in the near field, prior to marking for needle placement (66).
Chest CT
In chest infections, use of intravenous contrast is almost always indicated. Lower mA techniques (and kVp reduction in small children) can be used than in the abdomen, due to the high intrinsic contrast of lung parenchyma; further dose reduction is possible with follow-up of large lesions (abscess, empyema pockets) and for checking the position of chest tubes. Coronal reformats and 3D renditions (virtual bronchoscopy) are sometimes helpful tools prior to bronchoscopy or surgery.
Future Research
-
What is the cost-effectiveness of CT in management of empyema and parapneumonic effusions?
-
Can findings on imaging (plain radiography, ultrasound, CT) predict likelihood of success of various interventions for complications of pneumonia?
-
How can ultrasound, a non-irradiating modality, be utilized more in the evaluation of pulmonary infection and its complications?
-
What is the role of MR, a more expensive but non-ionizing modality, for evaluation of pulmonary infection complications (67)?
-
A prospective clinical trial to compare the benefits (including cost-effectiveness) of optimal image-guided intervention (with fibrinolysis) to early surgery (VATS) for the treatment of empyemas in children.
References
Chonmaitree T, Powell KR. Clin Pediatr (Phila) Jun 1983; 22(6):414–419.
Freij BJ, Kusmiesz H, Nelson JD, McCracken GH, Jr. Pediatr Infect Dis Nov–Dec 1984; 3(6):578–591.
Hardie W, Bokulic R, Garcia VF, Reising SF, Christie CD. Clin Infect Dis Jun 1996; 22(6):1057–1063.
Buckingham SC, King MD, Miller ML. Pediatr Infect Dis J Jun 2003; 22(6):499–504.
CDC.gov. Pneumonia among Children in Developing Countries. http://www.cdc.gov/ncidod/DBMD/diseaseinfo/pneumchilddevcount_t.htm
Jaffe A, Balfour-Lynn IM. Pediatr Pulmonol Aug 2005; 40(2):148–156.
Kuhn J. Caffey’s Pediatric Diagnostic Imaging, Vol I, 10 ed. Philadelphia: Mosby, 2004.
Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Bull World Health Organ May 2008; 86(5):408–416.
Murphy TF, Henderson FW, Clyde WA Jr, Collier AM, Denny FW. Am J Epidemiol Jan 1981; 113(1):12–21.
Margolis P, Gadomski A. JAMA Jan 28, 1998; 279(4):308–313.
Sawicki GS, Lu FL, Valim C, Cleveland RH, Colin AA. Eur Respir J Jan 23, 2008.
Barnes NP, Hull J, Thomson AH. Pediatr Pulmonol Feb 2005; 39(2):127–134.
Kurt BA, Winterhalter KM, Connors RH, Betz BW, Winters JW. Pediatrics Sep 2006; 118(3):e547–e553.
Vilar J, Domingo ML, Soto C, Cogollos J. Eur J Radiol Aug 2004; 51(2):102–113.
Kilbane BJ, Reynolds SL. Pediatr Emerg Care Feb 2008; 24(2):109–114; quiz 115–107.
Buescher ES. Curr Opin Pediatr Feb 2005; 17(1):67–70.
Franquet T. Eur Respir J Jul 2001; 18(1):196–208.
Shoham Y, Dagan R, Givon-Lavi N et al. Pediatrics May 2005; 115(5):1213–1219.
Palafox M, Guiscafre H, Reyes H, Munoz O, Martinez H. Arch Dis Child Jan 2000; 82(1):41–45.
Graffelman AW, le Cessie S, Knuistingh Neven A, Wilemssen FE, Zonderland HM, van den Broek PJ. J Fam Pract Jun 2007; 56(6):465–470.
McCracken GH, Jr. Pediatr Infect Dis J Sep 2000; 19(9):924–928.
Ostapchuk M, Roberts DM, Haddy R. Am Fam Physician Sep 1, 2004; 70(5):899–908.
Swingler GH, Hussey GD, Zwarenstein M. Lancet Feb 7, 1998; 351(9100):404–408.
Alario AJ, McCarthy PL, Markowitz R, Kornguth P, Rosenfield N, Leventhal JM. J Pediatr Aug 1987; 111(2):187–193.
Barnett ED Klein JO. Infectious Diseases of the Fetus and Nerborn Infant, 6th ed. Philadelphia: Elsevier Saunders Company, 2006.
Okimoto N, Yamato K, Kurihara T et al. Respirology May 2006; 11(3):322–324.
McIntosh K. N Engl J Med Feb 7 2002; 346(6):429–437.
Turner RB, Lande AE, Chase P, Hilton N, Weinberg D. J Pediatr Aug 1987; 111(2):194–200.
McCarthy PL. Curr Opin Pediatr Oct 1996; 8(5):427–429.
Virkki R, Juven T, Rikalainen H, Svedstrom E, Mertsola J, et al. Thorax May 2002; 57(5):438–441.
Korppi M, Kiekara O, Heiskanen-Kosma T, Soimakallio S. Acta Paediatr Apr 1993; 82(4):360–363.
King S, Thomson A. Br Med Bull 2002; 61:203–214.
Slater M, Krug SE. Emerg Med Clin North Am Feb 1999; 17(1):97-126, viii-ix.
Haney PJ, Bohlman M, Sun CC. Am J Roentgenol Jul 1984; 143(1):23–26.
Bachur R, Perry H, Harper MB. Ann Emerg Med Feb 1999; 33(2):166–173.
Swingler GH, Zwarenstein M. Cochrane Database Syst Rev 2005(3):CD001268.
Rigsby CK, Strife JL, Johnson ND, Atherton HD, Pommersheim W, Kotagal UR. Pediatr Radiol May 2004; 34(5):379–383.
Lamme T, Nijhout M, Cadman D et al. CMAJ Feb 15, 1986; 134(4):353–356.
Patenaude Y, Blais C, Leduc CP. Invest Radiol Jan 1995; 30(1):44–48.
Roback MG, Dreitlein DA. Pediatr Emerg Care Jun 1998; 14(3):181–184.
Perlstein PH, Kotagal UR, Bolling C et al. Pediatrics Dec 1999; 104(6):1334–1341.
Graffelman AW, Willemssen FE, Zonderland HM, Neven AK, Kroes AC, van den Broek PJ. Br J Gen Pract Feb 2008; 58(547):93–97.
Winer-Muram HT, Rubin SA, Kauffman WM et al. Clin Radiol Dec 1995; 50(12):842–847.
Winer-Muram HT, Rubin SA, Fletcher BD et al. Radiology Oct 1994; 193(1):127–133.
Winer-Muram HT, Arheart KL, Jennings SG, Rubin SA, Kauffman WM, Slobod KS. Radiology Sep 1997; 204(3):643–649.
Mori M, Galvin JR, Barloon TJ, Gingrich RD, Stanford W. Radiology Mar 1991; 178(3):721–726.
Kuhlman JE, Fishman EK, Burch PA, Karp JE, Zerhouni EA, Siegelman SS. Chest Jul 1987; 92(1):95–99.
Katz DS, Leung AN. Clin Chest Med Sep 1999; 20(3):549–562.
Janzen DL, Padley SP, Adler BD, Muller NL. Clin Radiol Mar 1993; 47(3):159–165.
Donnelly LF, Klosterman LA. Am J Roentgenol Jun 1998; 170(6):1627–1631.
Tan Kendrick AP, Ling H, Subramaniam R, Joseph VT. Pediatr Radiol Jan 2002; 32(1):16–21.
Donnelly LF, Klosterman LA. Am J Roentgenol Jul 1998; 171(1):253–256.
Heussel CP, Kauczor HU, Heussel G, Fischer B, Mildenberger P, et al. Am J Roentgenol Nov 1997; 169(5):1347–1353.
Barloon TJ, Galvin JR, Mori M, Stanford W, Gingrich RD. Chest Apr 1991; 99(4):928–933.
Ramnath RR, Heller RM, Ben-Ami T et al. Pediatrics Jan 1998; 101(1 Pt 1):68–71.
Kearney SE, Davies CW, Davies RJ, Gleeson FV. Clin Radiol Jul 2000; 55(7):542–547.
Donnelly LF, Klosterman LA. Am J Roentgenol Jul 1997; 169(1):179–182.
Donnelly LF. Radiol Clin North Am Mar 2005; 43(2):253–265.
Tu CY, Hsu WH, Hsia TC et al. Chest Oct 2004; 126(4):1274–1280.
Mitri RK, Brown SD, Zurakowski D et al. Pediatrics Sep 2002; 110(3):e37.
Shoseyov D, Bibi H, Shatzberg G et al. Chest Mar 2002; 121(3):836–840.
Cameron R, Davies HR. Cochrane Database Syst Rev 2008(2):CD002312.
Avansino JR, Goldman B, Sawin RS, Flum DR. Pediatrics Jun 2005; 115(6):1652–1659.
Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Thorax Apr 2002; 57(4):343–347.
Luh SP, Chou MC, Wang LS, Chen JY, Tsai TP. Chest Apr 2005; 127(4):1427–1432.
Coley BD. Radiol Clin North Am Mar 2005; 43(2):405–418.
Coskun A, Koc A, Yikilmaz A. Comparison of MRI with short imaging sequences and CXR for evaluation of pneumonia in pediatric patients. Program and abstracts of the Radiological Society of North America 93rd Scientific Assembly and Annual Meeting. Chicago:2007.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Choy, G., Yager, P.H., Noviski, N., Westra, S.J. (2010). Imaging of Chest Infections in Children. In: Medina, L., Applegate, K., Blackmore, C. (eds) Evidence-Based Imaging in Pediatrics. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-0922-0_27
Download citation
DOI: https://doi.org/10.1007/978-1-4419-0922-0_27
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-0921-3
Online ISBN: 978-1-4419-0922-0
eBook Packages: MedicineMedicine (R0)