Abstract
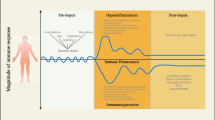
Septic syndromes represent a major although largely under-recognized healthcare problem worldwide accounting for thousands of deaths every year [1–3]. Mortality remains high ranging from 20 % for sepsis to over 50 % for septic shock despite almost 20 years of anti-inflammatory clinical trials [1–3]. The inability of these therapies to mitigate the devastating effects of this condition indicates that the initial hypotheses for sepsis pathophysiology may have been misconstrued or inadequately addressed. Two major explanations have been proposed: 1) Septic patients have mainly been treated as a group despite the extreme heterogeneity characterizing this population [1]; 2) The postulate that death after sepsis is solely due to an overwhelming pro-inflammatory immune response may actually be inaccurate [1, 3]. Indeed, several lines of evidence have now established that death from septic shock is probably due to the effect of distinct mechanisms over time [1–3]. Early in the course of the disease, a massive release of inflammatory mediators (normally designed to trigger an immune response against pathogens) is occurring that may be responsible for organ dysfunction and hypoperfusion [1, 3]. Concomitantly, the body develops compensatory mechanisms to prevent overwhelming inflammation and dampen an overzealous anti-infectious response [1–3]. These negative feedback mechanisms, although having protective effects during the first initial hours, may paradoxically become deleterious as they persist over time leading to immune paralysis (Fig. 1) [1, 3]. Indeed, considerable clinical and experimental evidence indicates that patients rapidly present with numerous compromised immune functions [1, 3].

Simplified description of systemic pro- and anti-inflammatory immune responses over time after septic shock. Dashed lines: pro- or anti-inflammatory responses; bold line: result at the systemic level. The shift from a pro-inflammatory to an anti-inflammatory immune response predominant at the systemic level likely occurs within 24 hours after the diagnosis of shock.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150
Remick DG (2007) Pathophysiology of sepsis. Am J Pathol 170: 1435–1444
Monneret G, Venet F, Pachot A, Lepape A (2008) Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 14: 64–78
Cavaillon JM, Adib-Conquy M (2007) Determining the degree of immunodysregulation in sepsis. Contrib Nephrol 156: 101–111
Venet F, Tissot S, Debard AL, et al (2007) Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: Correlation with severity and secondary septic shock. Crit Care Med 35: 1910–1917
Monneret G, Finck ME, Venet F, et al (2004) The anti-inflammatory response dominates after septic shock: Association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 95: 193–198
Oberholzer A, Oberholzer C, Moldawer LL (2002) Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med 30: S58–S63
Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J Infect Dis 181: 176–180
Williams TE, Ayala A, Chaudry IH (1997) Inducible macrophage apoptosis following sepsis is mediated by cysteine protease activation and nitric oxide release. J Surg Res 70: 113–118
Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A (2005) Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol 78: 325–337
Adrie C, Bachelet M, Vayssier-Taussat M, et al (2001) Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med 164: 389–395
Venet F, Pachot A, Debard AL, et al (2006) Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent. mechanism. J Immunol 177: 6540–6547
Kalechman Y, Gafter U, Gal R, et al (2002) Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: dependence on timing of immunomodulating intervention. J Immunol 169: 384–392
Polk HC Jr, Cheadle WG, Livingston DH, et al (1992) A randomized prospective clinical trial to determine the efficacy of interferon-gamma in severely injured patients. Am J Surg 163: 191–196
Dries DJ, Jurkovich GJ, Maier RV, et al (1994) Effect of interferon gamma on infection-related death in patients with severe injuries. A randomized, double-blind, placebo-controlled trial. Arch Surg 129: 1031–1041
Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW (2002) A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 166: 138–143
Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR (2005) Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest 127: 2139–2150.
Venet F, Chung CS, Monneret G, et al (2008) Regulatory T cell populations in sepsis and trauma. J Leukoc Biol 83: 523–535
Lederer JA, Rodrick ML, Mannick JA (1999) The effects of injury on the adaptive immune response. Shock 11: 153–159
Bandyopadhyay G, De A, Laudanski K, et al (2007) Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med 35: 794–801
Venet F, Bohe J, Debard AL, Bienvenu J, Lepape A, Monneret G (2005) Both percentage of gammadelta T lymphocytes and CD3 expression are reduced during septic shock. Crit Care Med 33: 2836–2840
Monneret G, Debard AL, Venet F, et al (2003) Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med 31: 2068–2071
Hotchkiss RS, Tinsley KW, Swanson PE, et al (2002) Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 168: 2493–2500
Hotchkiss RS, Swanson PE, Freeman BD, et al (1999) Apoptotic cell death in patients with sepsis, shock and multiple organ dysfunction. Crit Care Med 27: 1230–1251
Hotchkiss RS, Tinsley KW, Swanson PE, et al (2001) Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. bl]J Immunol 166: 6952–6963.
Le Tulzo Y, Pangault C, Gacouin A, et al (2002) Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18: 487–494
Bilbault P, Lavaux T, Lahlou A, et al (2004) Transient Bcl-2 gene down-expression in circulating mononuclear cells of severe sepsis patients who died despite appropriate intensive care. Intensive Care Med 30: 408–415
Bilbault P, Lavaux T, Launoy A, et al (2007) Influence of drotrecogin alpha (activated) infusion on the variation of Bax/Bcl-2 and Bax/Bcl-xl ratios in circulating mononuclear cells: a cohort study in septic shock patients. Crit Care Med 35: 69–75
Greineder CF, Nelson PW, Dressel AL, Erba HP, Younger JG (2007) In vitro and in silico analysis of annexin V binding to lymphocytes as a biomarker in emergency department sepsis studies. Acad Emerg Med 14: 763–771
Alpdogan O, van den Brink MR (2005) IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol 26: 56–64
Scumpia PO, Delano MJ, Kelly-Scumpia KM, et al (2007) Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood 110: 3673–3681
Turgeon AF, Hutton B, Fergusson DA, et al (2007) Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 146: 193–203
Weaver JG, Rouse MS, Steckelberg JM, Badley AD (2004) Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J 18: 1185–1191
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Venet, F., Lepape, A., Monneret, G. (2009). Monitoring Immune Dysfunction in Septic Patients: Toward Tailored Immunotherapy. In: Vincent, JL. (eds) Intensive Care Medicine. Springer, New York, NY. https://doi.org/10.1007/978-0-387-92278-2_8
Download citation
DOI: https://doi.org/10.1007/978-0-387-92278-2_8
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-92277-5
Online ISBN: 978-0-387-92278-2
eBook Packages: MedicineMedicine (R0)