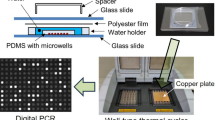
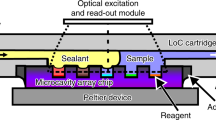
Abstract
Miniaturized integrated DNA analysis systems offer the potential to provide unprecedented advances in cost and speed relative to current benchtop-scale instrumentation by allowing rapid bioanalysis assays to be performed in a portable self contained device format that can be inexpensively mass-produced. The polymerase chain reaction (PCR) has been a natural focus of many of these miniaturization efforts, owing to its capability to efficiently replicate target regions of interest from small quantities template DNA. Scale-down of PCR has proven to be particularly challenging, however, due to an unfavorable combination of relatively severe temperature extremes (resulting in the need to repeatedly heat minute aqueous sample volumes to temperatures in the vicinity of 95°C with minimal evaporation) and high surface area to volume conditions imposed by nanoliter reactor geometries (often leading to inhibition of the reaction by nonspecific adsorption of reagents at the reactor walls). Despite these daunting challenges, considerable progress has been made in the development of microfluidic devices capable of performing increasingly sophisticated PCR-based bioassays. This chapter reviews the progress that has been made to date and assesses the outlook for future advances.
Chapter PDF
Similar content being viewed by others
Keywords
- Polymerase Chain Reaction
- Polymerase Chain Reaction Reactor
- Microfluidic Chip
- Polymerase Chain Reaction Reagent
- Polymerase Chain Reaction Chip
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Collins FS, Green ED, Guttmacher AE et al. A vision for the future of genomics research. Nature 2003; 422:835–847.
Sauer S, Lange BMH, Gobom J et al. Miniaturization in functional genomics and proteomics. Nat Rev Gene 2005; 6:465–476.
Syvänen A-C. Toward genome-wide SNP genotyping. Nat Gene 2005; 37:55–59.
Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations and future applications in acute-care settings. Lancet Infect Dis 2004; 4:337–348.
Cantor CR, Smith CL. Genomics: The science and technology behind the human genome project. New York: Wiley Interscience; 1999.
Spitzack KD, Ugaz VM. Polymerase chain reaction in miniaturized systems: big progress in little devices. In: Minteer SD, ed. Microfluidic Techniques: Reviews and Protocols. Vol 321. Totowa, New Jersey, USA: Humana Press; 2005: Chapter 10.
Belgrader P, Elkin CJ, Brown SB et al. A reusable flow-through polymerase chain reaction instrument for the continuous monitoring of infectious biological agents. Anal Chem 2003; 75:3446–3450.
Ebmeier RJ, Whitney SE, Sarkar A et al. Ranque-Hilsch vortex tube thermocycler for fast DNA amplification and real-time optical detection. Rev Sci Instrum 2004; 75:5356–5359.
Friedman NA, Meldrum DR. Capillary tube resistive thermocycling. Anal Chem 1998; 70:2997–3002.
Soper SA, Ford SM, Xu YC et al. Nanoliter-scale sample preparation methods directly coupled to polymethylmethacrylate-based microchips and gel-filled capillaries for the analysis of oligonucleotides. J Chromatogr A 1999; 853:107–120.
Swerdlow H, Jones BJ, Wittwer CT. Fully automated DNA reaction and analysis in a fluidic capillary instrument. Anal Chem 1997; 69:848–855.
Wittwer CT, Fillmore GC, Garling DJ. Minimizing the time required for DNA amplification by efficient heat transfer to small samples. Anal Biochem 1990; 186:328–331.
Wittwer CT, Fillmore GC, Hillyard DR. Automated polymerase chain-reaction in capillary tubes with hot air. Nucleic Acids Res 1989; 17:4353–4357.
Zhang NY, Tan HD, Yeung ES. Automated and integrated system for high-throughput DNA genotyping directly from blood. Anal Chem 1999; 71:1138–1145.
Erickson D, Li DQ. Integrated microfluidic devices. Anal Chim Acta 2004; 507:11–26.
Handal MI, Ugaz VM. DNA mutation detection and analysis using miniaturized microfluidic systems. Expert Rev Mol Diagn 2006; 6:29–38.
Kelly RT, Woolley AT. Microfluidic systems for integrated, high-throughput DNA analysis. Anal Chem 2005; 77:96A–102A.
Kricka LJ, Wilding P. Microchip PCR. Anal Bioanal Chem 2003; 377:820–825.
Lagally ET, Mathies RA. Integrated genetic analysis microsystems. J Phys D Appl Phys 2004; 37: R245–R261.
Lagally ET, Soh HT. Integrated genetic analysis microsystems. Critical Reviews in Solid State and Materials Sciences 2005; 30:207–233.
Roper MG, Easley CJ, Landers JP. Advances in polymerase chain reaction on microfluidic chips. Anal Chem 2005; 77:3887–3894.
Soper SA, Brown K, Ellington A et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens Bioelectron 2006; 21:1932–1942.
Wilding P. Nucleic acid amplification in microchips. In: Cheng J, Kricka LJ, eds. Biochip Technology. New York: Taylor & Francis Books, Inc.; 2003:173–184.
Zhang CS, Xu JL, Ma WL et al. PCR microfluidic devices for DNA amplification. Biotechnol Adv 2006; 24:243–284.
Woolley AT, Hadley D, Landre P et al. Functional integration of PCR amplification and capillary electrophoresis in a microfabricated DNA analysis device. Anal Chem 1996; 68:4081–4086.
Lagally ET, Simpson PC, Mathies RA. Monolithic integrated microfluidic DNA amplification and capillary electrophoresis analysis system. Sens Actuators B Chem 2000; 63:138–146.
Lagally ET, Medintz I, Mathies RA. Single-molecule DNA amplification and analysis in an integrated microfluidic device. Anal Chem 2001; 73:565–570.
Lagally ET, Emrich CA, Mathies RA. Fully integrated PCR-capillary electrophoresis microsystem for DNA analysis. Lab Chip 2001; 1:102–107.
Lagally ET, Scherer JR, Blazej RG et al. Integrated portable genetic analysis microsystem for pathogen/infectious disease detection. Anal Chem 2004; 76:3162–3170.
Dunn WC, Jacobson SC, Waters LC et al. PCR amplification and analysis of simple sequence length polymorphisms in mouse DNA using a single microchip device. Anal Biochem 2000; 277:157–160.
Waters LC, Jacobson SC, Kroutchinina N et al. Multiple sample PCR amplification and electrophoretic analysis on a microchip. Anal Chem 1998; 70:5172–5176.
Waters LC, Jacobson SC, Kroutchinina N et al. Microchip device for cell lysis, multiplex PCR amplification and electrophoretic sizing. Anal Chem 1998; 70:158–162.
Khandurina J, McKnight TE, Jacobson SC et al. Integrated system for rapid PCR-based DNA analysis in microfluidic devices. Anal Chem 2000; 72:2995–3000.
Zhou ZM, Liu DY, Zhong RT et al. Determination of SARS-coronavirus by a microfluidic chip system. Electrophoresis 2004; 25:3032–3039.
Hong JW, Fujii T, Seki M et al. Integration of gene amplification and capillary gel electrophoresis on a polydimethylsiloxane-glass hybrid microchip. Electrophoresis 2001; 22:328–333.
Rodriguez I, Lesaicherre M, Tie Y et al. Practical integration of polymerase chain reaction amplification and electrophoretic analysis in microfluidic devices for genetic analysis. Electrophoresis 2003; 24:172–178.
Koh CG, Tan W, Zhao M et al. Integrating polymerase chain reaction, valving and electrophoresis in a plastic device for bacterial detection. Anal Chem 2003; 75:4591–4598.
Ferrance JP, Wu QR, Giordano B et al. Developments toward a complete micro-total analysis system for Duchenne muscular dystrophy diagnosis. Anal Chim Acta 2003; 500:223–236.
Easley CJ, Karlinsey JM, Landers JP. On-chip pressure injection for integration of infrared-mediated DNA amplification with electrophoretic separation. Lab Chip 2006; 6:601–610.
Wilding P, Kricka LJ, Cheng J et al. Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal Biochem 1998; 257:95–100.
Yuen PK, Kricka LJ, Fortina P et al. Microchip module for blood sample preparation and nucleic acid amplification reactions. Genome Res 2001; 11:405–412.
Panaro NJ, Lou XJ, Fortina P et al. Micropillar array chip for integrated white blood cell isolation and PCR. Biomol Eng 2005; 21:157–162.
Lee CY, Lee GB, Lin JL et al. Integrated microfluidic systems for cell lysis, mixing/pumping and DNA amplification. J Micromech Microeng 2005; 15:1215–1223.
Cady NC, Stelick S, Kunnavakkam MV et al. Real-time PCR detection of Listeria monocytogenes using an integrated microfluidics platform. Sens Actuators B Chem 2005; 107:332–341.
Anderson RC, Su X, Bogdan GJ et al. A miniature integrated device for automated multistep genetic analysis. Nucleic Acids Res 2000; 28:E60.
Liu RH, Yang J, Lenigk R et al. Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification and DNA microarray detection. Anal Chem 2004; 76:1824–1831.
Trau D, Lee TMH, Lao AIK et al. Genotyping on a complementary metal oxide semiconductor silicon polymerase chain reaction chip with integrated DNA microarray. Anal Chem 2002; 74:3168–3173.
Liu YJ, Rauch CB, Stevens RL et al. DNA amplification and hybridization assays in integrated plastic monolithic devices. Anal Chem 2002; 74:3063–3070.
Burns MA, Johnson BN, Brahmasandra SN et al. An Integrated Nanoliter DNA Analysis Device. Science 1998; 282:484–487.
Pal R, Yang M, Lin R et al. An integrated microfluidic device for influenza and other genetic analyses. Lab Chip 2005; 5:1024–1032.
Blazej RG, Kumaresan P, Mathies RA. Microfabricated bioprocessor for integrated nanoliter-scale Sanger DNA sequencing. Proc Natl Acad Sci USA 2006; 103:7240–7245.
Krishnan M, Ugaz VM, Burns MA. PCR in a Rayleigh-Bénard convection cell. Science 2002; 298:793.
Ugaz VM, M.K. Novel convective flow based approaches for high-throughput PCR thermocycling. JALA 2004; 9:318–323.
Yariv E, Ben-Dov G, Dorfman K. Polymerase chain reaction in natural convection systems: A convection-diffusion-reaction model. Europhys Lett 2005; 71:1008–1014.
Krishnan M, Agrawal N, Burns MA et al. Reactions and fluidics in miniaturized natural convection systems. Anal Chem 2004; 76:6254–6265.
Braun D. PCR by thermal convection. Modern Physics Letters B 2004; 18:775–784.
Braun D, Goddard NL, Libchaber A. Exponential DNA replication by laminar convection. Phys Rev Lett 2003; 91:158103.
Hennig M, Braun D. Convective polymerase chain reaction around micro immersion heater. Appl Phys Lett 2005; 87:183901.
Wheeler EK, Benett W, Stratton P et al. Convectively driven polymerase chain reaction thermal cycler. Anal Chem 2004; 76:4011–4016.
Chen Z, Qian S, Abrams WR et al. Thermosiphon-based PCR reactor: experiment and modeling. Anal Chem 2004; 76:3707–3715.
Agrawal N, Hassan YA, Ugaz VM. A pocket-sized convective PCR thermocycler. Angew Chem Int Ed 2007; in press.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2007 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Ugaz, V.M. (2007). PCR in Integrated Microfluidic Systems. In: Liu, R.H., Lee, A.P. (eds) Integrated Biochips for DNA Analysis. Biotechnology Intelligence Unit. Springer, New York, NY. https://doi.org/10.1007/978-0-387-76759-8_7
Download citation
DOI: https://doi.org/10.1007/978-0-387-76759-8_7
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-76758-1
Online ISBN: 978-0-387-76759-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)