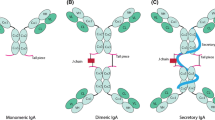
The production of monoclonal antibodies and the development of recombinant antibody technology have made antibodies one of the largest classes of drugs in development for prophylactic, therapeutic and diagnostic purposes. Currently, all of the Food and Drug Administration (FDA)- approved antibodies are immunoglobulin Gs (IgGs). However, more than 95%of the infections are initiated at the mucosal surfaces, where IgA is the primary immune effector antibody.
Chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
References
Atkin, J. D., Pleass, R. J., Owens, R. J., and Woof, J. M. (1996). Mutagenesis of the human IgA1 heavy chain tailpiece that prevents dimer assembly. J. Immunol. 157:156–159.
Baenziger, J. U. (1979). Structure of the oligosaccharide of human J chain. J. Biol. Chem. 254:4063–4071.
Bakker, H., Bardor, M., Molthoff, J. W., Gomord, V., Elbers, I., Stevens, L. H., Jordi, W., Lommen, A., Faye, L., Lerouge, P., and Bosch, D. (2001). Galactose-extended glycans of antibodies produced by transgenic plants. Proc. Natl. Acad. Sci. USA 98:2899–2904.
Basset, C., Devauchelle, V., Durand, V., Jamin, C., Pennec, Y. L., Youinou, P., and Dueymes, M. (1999). Glycosylation of immunoglobulin A influences its receptor binding. Scand. J. Immunol. 50:572–579.
Batten, M. R., Senior, B. W., Kilian, M., and Woof, J. M. (2003). Amino acid sequence requirements in the hinge of human immunoglobulin A1 (IgA1) for cleavage by streptococcal IgA1 proteases. Infect. Immun. 71:1462–1469.
Baum, R. P., Niesen, A., Hertel, A., Nancy, A., Hess, H., Donnerstag, B., Sykes, T. R., Sykes, C. J., Suresh, M. R., Noujaim, A. A., et al. (1994). Activating anti-idiotypic human anti-mouse antibodies for immunotherapy of ovarian carcinoma. Cancer 73:1121–1125.
Berdoz, J., Blanc, C. T., Reinhardt, M., Kraehenbuhl, J. P., and Corthesy, B. (1999). In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc. Natl. Acad. Sci. USA 96:3029–3034.
Berdoz, J., and Corthesy, B. (2004). Human polymeric IgA is superior to IgG and single-chain Fv of the same monoclonal specificity to inhibit urease activity associated with Helicobacter pylori. Mol. Immunol. 41:1013–1022.
Berdoz, J., Monath, T. P., and Kraehenbuhl, J. P. (1995). Specific amplification by PCR of rearranged genomic variable regions of immunoglobulin genes from mouse hybridoma cells. PCR Methods Appl. 4:256–264.
Biswas, S., Saxena, Q. B., Roy, A., and Kubilan, L. (1995). Naturally occurring Plasmodium specific IgA antibodies in humans from a malaria endemic area. J. Biosci. 20:453–460.
Boel, E., Verlaan, S., Poppelier, M. J., Westerdaal, N. A., Van Strijp, J. A., and Logtenberg, T. (2000). Functional human monoclonal antibodies of all isotypes constructed from phage display library-derived single-chain Fv antibody fragments. J. Immunol. Methods 239:153–166.
Braathen, R., Sorensen, V., Brandtzaeg, P., Sandlie, I., and Johansen, F. E. (2002). The carboxyl-terminal domains of IgA and IgM direct isotype-specific polymerization and interaction with the polymeric immunoglobulin receptor. J. Biol. Chem. 277:42, 755–42, 762.
Brandtzaeg, P., and Prydz, H. (1984). Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311:71–73.
Breitz, H. B., Weiden, P. L., Vanderheyden, J. L., Appelbaum, J. W., Bjorn, M. J., Fer, M. F., Wolf, S. B., Ratliff, B. A., Seiler, C. A., Foisie, D. C., et al. (1992). Clinical experience with rhenium-186-labeled monoclonal antibodies for radioimmunotherapy: results of phase I trials. J. Nucl. Med. 33:1099–1109.
Brooks, D., Taylor, C., Dos Santos, B., Linden, H., Houghton, A., Hecht, T. T., Kornfeld, S., and Taetle, R. (1995). Phase Ia trial of murine immunoglobulin A antitransferrin receptor antibody 42/6. Clin. Cancer Res. 1:1259–1265.
Bruggemann, M., Williams, G. T., Bindon, C. I., Clark, M. R., Walker, M. R., Jefferis, R., Waldmann, H., and Neuberger, M. S. (1987). Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J. Exp. Med. 166:1351–1361.
Buchegger, F., Pfister, C., Fournier, K., Prevel, F., Schreyer, M., Carrel, S., and Mach, J. P. (1989). Ablation of human colon carcinoma in nude mice by 131I-labeled monoclonal anti-carcinoembryonic antigen antibody F(ab’) 2 fragments. J. Clin. Invest. 83:1449–1456.
Burtin, P., von Kleist, S., Sabine, M. C., and King, M. (1973). Immunohistological localization of carcinoembryonic antigen and nonspecific cross-reacting antigen in gastrointestinal normal and tumoral tissues. Cancer Res. 33:3299–3305.
Butters, T. D., Hughes, R. C., and Vischer, P. (1981). Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim. Biophys. Acta 640:672–686.
Campbell, M. J., Zelenetz, A. D., Levy, S., and Levy, R. (1992). Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol. Immunol. 29:193–203.
Carayannopoulos, L., Hexham, J. M., and Capra, J. D. (1996). Localization of the binding site for the monocyte immunoglobulin (Ig) A-Fc receptor (CD89) to the domain boundary between Calpha2 and Calpha3 in human IgA1. J. Exp. Med. 183:1579–1586.
Carayannopoulos, L., Max, E. E., and Capra, J. D. (1994). Recombinant human IgA expressed in insect cells. Proc. Natl. Acad. Sci. USA 91:8348–8352.
Casadevall, A., Dadachova, E., and Pirofski, L. A. (2004). Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695–703.
Chargelegue, D., Vine, N. D., van Dolleweerd, C. J., Drake, P. M., and Ma, J. K. (2000). A murine monoclonal antibody produced in transgenic plants with plant-specific glycans is not immunogenic in mice. Transgenic Res. 9:187–194.
Chintalacharuvu, K. R., Chuang, P. D., Dragoman, A., Fernandez, C. Z., Qiu, J., Plaut, A. G., Trinh, K. R., Gala, F. A., and Morrison, S. L. (2003). Cleavage of the human immunoglobulin A1 (IgA1) hinge region by IgA1 proteases requires structures in the Fc region of IgA. Infect. Immun. 71:2563–2570.
Chintalacharuvu, K. R., and Morrison, S. L. (1996). Residues critical for H-L disulfide bond formation in human IgA1 and IgA2. J. Immunol. 157:3443–3449.
Chintalacharuvu, K. R., and Morrison, S. L. (1997). Production of secretory immunoglobulin A by a single mammalian cell. Proc. Natl. Acad. Sci. USA 94:6364–6368.
Chintalacharuvu, K. R., Raines, M., and Morrison, S. L. (1994). Divergence of human alpha-chain constant region gene sequences. A novel recombinant alpha 2 gene. J. Immunol. 152:5299–5304.
Chintalacharuvu, K. R., Vuong, L. U., Loi, L. A., Larrick, J. W., and Morrison, S. L. (2001). Hybrid IgA2/IgG1 antibodies with tailor-made effector functions. Clin. Immunol. 101:21–31.
Chintalacharuvu, K. R., Yu, L. J., Bhola, N., Kobayashi, K., Fernandez, C. Z., and Morrison, S. L. (2002). Cysteine residues required for the attachment of the light chain in human IgA2. J. Immunol. 169:5072–5077.
Chuang, P. D., and Morrison, S. L. (1997). Elimination of N-linked glycosylation sites from the human IgA1 constant region: effects on structure and function. J. Immunol. 158:724–732.
Clynes, R. A., Towers, T. L., Presta, L. G., and Ravetch, J. V. (2000). Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6:443–446.
Coloma, M. J., Hastings, A., Wims, L. A., and Morrison, S. L. (1992). Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J. Immunol. Methods 152:89–104.
Corthesy, B., Kaufmann, M., Phalipon, A., Peitsch, M., Neutra, M. R., and Kraehenbuhl, J. P. (1996). A pathogen-specific epitope inserted into recombinant secretory immunoglobulin A is immunogenic by the oral route. J. Biol. Chem. 271:33, 670–677.
Cottet, S., and Corthesy, B. (1997). Cellular processing limits the heterologous expression of secretory component in mammalian cells. Eur. J. Biochem. 246:23–31.
Crottet, P., and Corthesy, B. (1998). Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’) 2: A possible implication for mucosal defense. J. Immunol. 161:5445–5453.
Crottet, P., Cottet, S., and Corthesy, B. (1999). Expression, purification and biochemical characterization of recombinant murine secretory component: a novel tool in mucosal immunology. Biochem. J. 341(Pt. 2):299–306.
Dallas, S. D., and Rolfe, R. D. (1998). Binding of Clostridium difficile toxin A to human milk secretory component. J. Med. Microbiol. 47:879–888.
Davies, J., Jiang, L., Pan, L. Z., LaBarre, M. J., Anderson, D., and Reff, M. (2001). Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for Fc gamma RIII. Biotechnol. Bioeng. 74:288–294.
Davis, R., Schooley, K., Rasmussen, B., Thomas, J., and Reddy, P. (2000). Effect of PDI overexpression on recombinant protein secretion in CHO cells. Biotechnol. Prog. 16:736–743.
Dechant, M., Vidarsson, G., Stockmeyer, B., Repp, R., Glennie, M. J., Gramatzki, M., van De Winkel, J. G., and Valerius, T. (2002). Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood 100:4574–4580.
De Groot, N., Van Kuik-Romeijn, P., Lee, S. H., and De Boer, H. A. (2000). Increased immunoglobulin A levels in milk by over-expressing the murine polymeric immunoglobulin receptor gene in the mammary gland epithelial cells of transgenic mice. Immunology 101:218–224.
Devito, C., Broliden, K., Kaul, R., Svensson, L., Johansen, K., Kiama, P., Kimani, J., Lopalco, L., Piconi, S., Bwayo, J. J., et al. (2000a). Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170–5176.
Devito, C., Hinkula, J., Kaul, R., Lopalco, L., Bwayo, J. J., Plummer, F., Clerici, M., and Broliden, K. (2000b). Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14:1917–1920.
Elbers, I. J., Stoopen, G. M., Bakker, H., Stevens, L. H., Bardor, M., Molthoff, J. W., Jordi, W. J., Bosch, D., and Lommen, A. (2001). Influence of growth conditions and developmental stage on N-glycan heterogeneity of transgenic immunoglobulin G and endogenous proteins in tobacco leaves. Plant Physiol. 126:1314–1322.
Favre, L. I., Spertini, F., and Corthesy, B. (2003). Simplified procedure to recover recombinant antigenized secretory IgA to be used as a vaccine vector. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 786:143–151.
Fukuta, K., Abe, R., Yokomatsu, T., Omae, F., Asanagi, M., and Makino, T. (2000). Control of bisecting GlcNAc addition to N-linked sugar chains. J. Biol. Chem. 275:23, 456–461.
Galili, U. (1989). Abnormal expression of alpha-galactosyl epitopes in man. A trigger for autoimmune processes? Lancet 2:358–361.
Gavilondo-Cowley, J. V., Coloma, M. J., Vazquez, J., Ayala, M., Macias, A., Fry, K. E., and Larrick, J. W. (1990). Specific amplification of rearranged immunoglobulin variable region genes from mouse hybridoma cells. Hybridoma 9:407–417.
Gillies, S. D., Lo, K. M., and Wesolowski, J. (1989). High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J. Immunol. Methods 125:191–202.
Griffiss, G. M., and Goroff, D. K. (1983). IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J. Immunol. 130:2882–2885.
Gupta, N., Arthos, J., Khazanie, P., Steenbeke, T. D., Censoplano, N. M., Chung, E. A., Cruz, C. C., Chaikin, M. A., Daucher, M., Kottilil, S., Mavilio, D., Schuck, P., Sun, P. D., Rabin, R. L., Radaev, S., Van Ryk, D., Cicala, C., and Fauci, A. S. (2005). Targeted lysis of HIV-infected cells by natural killler cells armed and triggered by a recombinant immunoglobulin fusion protein: Implication for immunotherapy. Vaccine 332:491–497.
Hemming, V. G., Rodriguez, W., Kim, H. M., Brandt, C. D., Parrott, R. H., Burch, B., Prince, G. A., Baron, P. A., Fink, R. J., and Reaman, G. (1987). Intravenous immunoglobulin treatment of respiratory syncytial virus infection in infants and young children. Antimicrob. Agents Chemother. 31:1882–1886.
Herr, A. B., Ballister, E. R., and Bjorkman, P. J. (2003). Insights into IgA-mediated immune response from the cystal structures of human Fc alpha RI and its complex with IgA1-Fc. Nature 423:614–620.
Hexham, J. M., White, K. D., Carayannopoulos, L. N., Mandecki, W., Brisette, R., Yang, Y. S., and Capra, J. D. (1999). A human immunoglobulin (Ig) A C alpha 3 domain motif directs polymeric Ig receptor-mediated secretion. J. Exp. Med. 189:747–752.
Hills, A. E., Patel, A., Boyd, P., and James, D. C. (2001). Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells. Biotechnol. Bioeng. 75:239–251.
Hirt, R. P., Hughes, G. J., Frutiger, S., Michetti, P., Perregaux, C., Poulain-Godefroy, O., Jeanguenat, N., Neutra, M. R., and Kraehenbuhl, J. P. (1993). Transcytosis of the polymeric Ig receptor requires phosphorylation of serine 664 in the absence but not the presence of dimeric IgA. Cell 74:245–255.
Houdebine, L. M. (2000). Transgenic animal bioreactors. Transgenic Res. 9:305–320.
Hughes, G. J., Reason, A. J., Savoy, L., Jaton, J., and Frutiger-Hughes, S. (1999). Carbohydrate moieties in human secretory component. Biochim. Biophys. Acta 1434:86–93.
Huls, G., Heijnen, I. A., Cuomo, E., van der Linden, J., Boel, E., van de Winkel, J. G., and Logtenberg, T. (1999a). Antitumor immune effector mechanisms recruited by phage display-derived fully human IgG1 and IgA1 monoclonal antibodies. Cancer Res. 59:5778–5784.
Huls, G. A., Heijnen, I. A., Cuomo, M. E., Koningsberger, J. C., Wiegman, L., Boel, E., van der Vuurst de Vries, A. R., Loyson, S. A., Helfrich, W., van Berge Henegouwen, G. P., van Meijer, M., de Kruif, J., and Logtenberg, T. (1999b). A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nat. Biotechnol. 17:276–281.
Janoff, E. N., Fasching, C., Orenstein, J. M., Rubins, J. B., Opstad, N. L., and Dalmasso, A. P. (1999). Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Invest. 104:1139–1147.
Jarvis, D. L., and Finn, E. E. (1995). Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology 212:500–511.
Jarvis, D. L., and Finn, E. E. (1996). Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat. Biotechnol. 14:1288–1292.
Johansen, F. E., Braathen, R., and Brandtzaeg, P. (2001). The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J. Immunol. 167:5185–5192.
Johansen, F. E., Natvig Norderhaug, I., Roe, M., Sandlie, I., and Brandtzaeg, P. (1999). Recombinant expression of polymeric IgA: Incorporation of J chain and secretory component of human origin. Eur. J. Immunol. 29:1701–1708.
Kaul, R., Trabattoni, D., Bwayo, J. J., Arienti, D., Zagliani, A., Mwangi, F. M., Kariuki, C., Ngugi, E. N., MacDonald, K. S., Ball, T. B., Clerici, M., and Plummer, F. A. (1999). HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23–29.
Knight, K. L., Suter, M., and Becker, R. S. (1988). Genetic engineering of bovine Ig. Construction and characterization of hapten-binding bovine/murine chimeric IgE, IgA, IgG1, IgG2, and IgG3 molecules. J. Immunol. 140:3654–3659.
Kozlowski, P. A., and Neutra, M. R. (2003). The role of mucosal immunity in preventing of HIV transmission. Curr. Mol. Med. 3:217–228.
Larrick, J. W., Danielsson, L., Brenner, C. A., Abrahamson, M., Fry, K. E., and Borrebaeck, C. A. (1989). Rapid cloning of rearranged immunoglobulin genes from human hybridoma cells using mixed primers and the polymerase chain reaction. Biochem. Biophys. Res. Commun. 160:1250–1256.
Larrick, J. W., Yu, L., Naftzger, C., Jaiswal, S., and Wycoff, K. (2001). Production of secretory IgA antibodies in plants. Biomol. Eng. 18:87–94.
Lee, E. U., Roth, J., and Paulson, J. C. (1989). Alteration of terminal glycosylation sequences on N-linked oligosaccharides of Chinese hamster ovary cells by expression of beta-galactoside alpha 2, 6-sialyltransferase. J. Biol. Chem. 264:13, 848–855.
Leke, R. G., Ndansi, R., Southerland, N. J., Quakyi, I. A., and Taylor, D. W. (1992). Identification of anti-Plasmodium falciparum antibodies in human breast milk. Scand. J. Immunol. 11(Suppl.):17–22.
Lifely, M. R., Hale, C., Boyce, S., Keen, M. J., and Phillips, J. (1995). Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology 5:813–822.
Lindh, E. (1975). Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 114:284–286.
Lo, D., Pursel, V., Linton, P. J., Sandgren, E., Behringer, R., Rexroad, C., Palmiter, R. D., and Brinster, R. L. (1991). Expression of mouse IgA by transgenic mice, pigs and sheep. Eur. J. Immunol. 21:1001–1006.
Lullau, E., Heyse, S., Vogel, H., Marison, I., von Stockar, U., Kraehenbuhl, J. P., and Corthesy, B. (1996). Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J. Biol. Chem. 271:16, 300–16, 309.
Lund, J., Takahashi, N., Hindley, S., Tyler, R., Goodall, M., and Jefferis, R. (1993). Glycosylation of human IgG subclass and mouse IgG2b heavy chains secreted by mouse J558L transfectoma cell lines as chimeric antibodies. Hum. Antibodies Hybridomas 4:20–25.
Lund, J., Tanaka, T., Takahashi, N., Sarmay, G., Arata, Y., and Jefferis, R. (1990). A protein structural change in aglycosylated IgG3 correlates with loss of huFc gamma R1 and huFc gamma R111 binding and/or activation. Mol. Immunol. 27:1145–1153.
Ma, J. K., Hiatt, A., Hein, M., Vine, N. D., Wang, F., Stabila, P., van Dolleweerd, C., Mostov, K., and Lehner, T. (1995). Generation and assembly of secretory antibodies in plants. Science 268:716–719.
Ma, J. K., Hikmat, B. Y., Wycoff, K., Vine, N. D., Chargelegue, D., Yu, L., Hein, M. B., and Lehner, T. (1998). Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4:601–606.
Mantis, N. J., Farrant, S. A., and Mehta, S. (2004). Oligosaccharide side chains on human secretory IgA serve as receptors for ricin. J. Immunol. 172:6838–6845.
Martin, B. M., Tsuji, S., LaMarca, M. E., Maysak, K., Eliason, W., and Ginns, E. I. (1988). Glycosylation and processing of high levels of active human glucocerebrosidase in invertebrate cells using a baculovirus expression vector. DNA 7:99–106.
Mattu, T. S., Pleass, R. J., Willis, A. C., Kilian, M., Wormald, M. R., Lellouch, A. C., Rudd, P. M., Woof, J. M., and Dwek, R. A. (1998). The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J. Biol. Chem. 273:2260–2272.
McKinney, K. L., Dilwith, R., and Belfort, G. (1995). Optimizing antibody production in batch hybridoma cell culture. J. Biotechnol. 40:31–48.
Merry, A. H., Morton, C., Bruce, J., Kerr, M., and Woof, J. M. (1992). Glycosylation of recombinant chimeric and human serum IgA1. Biochem. Soc. Trans. 20:92S.
Miller, L. H., Good, M. F., and Milon, G. (1994). Malaria pathogenesis. Science 264:1878–1883.
Monica, T. J., Goochee, C. F., and Maiorella, B. L. (1993). Comparative biochemical characterization of a human IgM produced in both ascites and in vitro cell culture. Biotechnology (NY) 11:512–515.
Monteiro, R. C., Kubagawa, H., and Cooper, C. (1990). Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in human. J. Exp. Med. 171:597–613.
Morton, H. C., Atkin, J. D., Owens, R. J., and Woof, J. M. (1993). Purification and characterization of chimeric human IgA1 and IgA2 expressed in COS and Chinese hamster ovary cells. J. Immunol. 151:4743–4752.
Motegi, Y., and Kita, H. (1998). Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J. Immunol. 161:4340–4346.
Nyberg, G. B., Balcarcel, R. R., Follstad, B. D., Stephanopoulos, G., and Wang, D. I. (1999). Metabolic effects on recombinant interferon-gamma glycosylation in continuous culture of Chinese hamster ovary cells. Biotechnol. Bioeng. 62:336–347.
Ogonah, O. W., Freedman, R. B., Jenkins, N., and Rooney, B. C. (1995). Analysis of human interferon-gamma glycoforms produced in baculovirus infected insect cells by matrix assisted laser desorption spectrometry. Biochem. Soc. Trans. 23:100S.
Orlandi, R., Gussow, D. H., Jones, P. T., and Winter, G. (1989). Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:3833–3837.
Otten, M. A., Rudolph, E., Dechant, M., Tuk, C. W., Reijmers, R. M., Beelen, R. H., van de Winkel, J. G., and van Egmond, M. (2005). Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J. Immunol. 174:5472–5480.
Phalipon, A., Cardona, A., Kraehenbuhl, J. P., Edelman, L., Sansonetti, P. J., and Corthesy, B. (2002). Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17:107–115.
Pleass, R. J., Dehal, P. K., Lewis, M. J., and Woof, J. M. (2003a). Limited role of charge matching in the interaction of human immunoglobulin A with the immunoglobulin A Fc receptor (Fc alpha RI) CD89. Immunology 109:331–335.
Pleass, R. J., Dunlop, J. I., Anderson, C. M., and Woof, J. M. (1999). Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human fcalpha receptor (FcalphaR) CD89. J. Biol. Chem. 274:23, 508–514.
Pleass, R. J., Ogun, S. A., McGuinness, D. H., van de Winkel, J. G., Holder, A. A., and Woof, J. M. (2003b). Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood 102:4424–4430.
Preston, M. J., Gerceker, A. A., Reff, M. E., and Pier, G. B. (1998). Production and characterization of a set of mouse-human chimeric immunoglobulin G (IgG) subclass and IgA monoclonal antibodies with identical variable regions specific for Pseudomonas aeruginosa serogroup O6 lipopolysaccharide. Infect. Immun. 66:4137–4142.
Prost, A. C., Menegaux, F., Langlois, P., Vidal, J. M., Koulibaly, M., Jost, J. L., Duron, J. J., Chigot, J. P., Vayre, P., Aurengo, A., Legrand, J. C., Rosselin, G., and Gespach, C. (1998). Differential transferrin receptor density in human colorectal cancer: A potential probe for diagnosis and therapy. Int. J. Oncol. 13:871–875.
Raju, T. S., Briggs, J. B., Borge, S. M., and Jones, A. J. (2000). Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10:477–486.
Raju, T. S., Briggs, J. B., Chamow, S. M., Winkler, M. E., and Jones, A. J. (2001). Glycoengineering of therapeutic glycoproteins: in vitro galactosylation and sialylation of glycoproteins with terminal N-acetylglucosamine and galactose residues. Biochemistry 40:8868–8876.
Ramirez, N., Rodriguez, M., Ayala, M., Cremata, J., Perez, M., Martinez, A., Linares, M., Hevia, Y., Paez, R., Valdes, R., et al. (2003). Expression and characterization of an anti-(hepatitis B surface antigen) glycosylated mouse antibody in transgenic tobacco (Nicotiana tabacum) plants and its use in the immunopurification of its target antigen. Biotechnol. Appl. Biochem. 38:223–230.
Renegar, K. B., Jackson, G. D., and Mestecky, J. (1998a). In vitro comparision of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J. Immunol. 160:1219–1223.
Rifai, A., Fadden, K., Morrison, S. L., and Chintalacharuvu, K. R. (2000). The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig) A1 and IgA2 isotypes. J. Exp. Med. 191:2171–2182.
Rindisbacher, L., Cottet, S., Wittek, R., Kraehenbuhl, J. P., and Corthesy, B. (1995). Production of human secretory component with dimeric IgA binding capacity using viral expression systems. J. Biol. Chem. 270:14, 220–14, 228.
Routier, F. H., Davies, M. J., Bergemann, K., and Hounsell, E. F. (1997). The glycosylation pattern of humanized IgGI antibody (D1.3) expressed in CHO cells. Glycoconj. J. 14:201–207.
Royle, L., Roos, A., Harvey, D. J., Wormald, M. R., van Gijlswijk-Janssen, D., Redwan el, R. M., Wilson, I. A., Daha, M. R., Dwek, R. A., and Rudd, P. M. (2003). Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem. 278:20, 140–153.
Russell, M. W., Reinholdt, J., and Kilian, M. (1998). Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: Inhibition of IgG-mediated complement activation. Eur. J. Immunol. 24:1211–1217.
Sandin, C., Linse, S., Areschoug, T., Woof, J. M., Reinholdt, J., and Lindahl, G. (2002). Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J. Immunol. 169:1357–1364.
Sauer, P. W., Burky, J. E., Wesson, M. C., Sternard, H. D., and Qu, L. (2000). A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol. Bioeng. 67:585–597.
Schneider, M., Marison, I. W., and von Stockar, U. (1996). The importance of ammonia in mammalian cell culture. J. Biotechnol. 46:161–185.
Schneiderman, R. D., Hanly, W. C., and Knight, K. L. (1989). Expression of 12 rabbit IgA C alpha genes as chimeric rabbit-mouse IgA antibodies. Proc. Natl. Acad. Sci. USA 86:7561–7565.
Schneiderman, R. D., Lint, T. F., and Knight, K. L. (1990). Activation of the alternative pathway of complement by twelve different rabbit-mouse chimeric transfectoma IgA isotypes. J. Immunol. 145:233–237.
Schwarze, J., Cieslewicz, G., Joetham, A., Sun, L. K., Sun, W. N., Chang, T. W., Hamelmann, E., and Gelfand, E. W. (1998). Antigen-specific immunoglobulin-A prevents increased airway responsiveness and lung eosinophilia after airway challenge in sensitized mice. Am. J. Respir. Crit. Care Med. 158:519–525.
Senior, B. W., Dunlop, J. I., Batten, M. R., Kilian, M., and Woof, J. M. (2000). Cleavage of a recombinant human immunoglobulin A2 (IgA2)–IgA1 hybrid antibody by certain bacterial IgA1 proteases. Infect. Immun. 68:463–469.
Senior, B. W., and Woof, J. M. (2005a). Effect of mutations in the human immunoglobulin A1 (IgA1) hinge on its susceptibility to cleavage by diverse bacterial IgA1 proteases. Infect. Immun. 73:1515–1522.
Senior, B. W., and Woof, J. M. (2005b). The influences of hinge length and composition on the susceptibility of human IgA to cleavage by diverse bacterial IgA1 proteases. J. Immunol. 174:7792–7799.
Shinohara, H., Fan, D., Ozawa, S., Yano, S., Van Arsdell, M., Viner, J. L., Beers, R., Pastan, I., and Fidler, I. J. (2000). Site-specific expression of transferrin receptor by human colon cancer cells directly correlates with eradication by antitransferrin recombinant immunotoxin. Int. J. Oncol. 17:643–651.
Sola, I., Castilla, J., Pintado, B., Sanchez-Morgado, J. M., Whitelaw, C. B., Clark, A. J., and Enjuanes, L. (1998). Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J. Virol. 72:3762–3772.
Stoll, T. S., Muhlethaler, K., von Stockar, U., and Marison, I. W. (1996). Systematic improvement of a chemically-defined protein-free medium for hybridoma growth and monoclonal antibody production. J. Biotechnol. 45:111–123.
Stubbe, H., Berdoz, J., Kraehenbuhl, J. P., and Corthesy, B. (2000). Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164:1952–1960.
Subramanian, K. N., Weismann, L. E., Rhodes, T., Ariagno, R., Sanchez, P. J., Steichen, J., Givner, L. B., Jennings, T. L., Top, F. H. J., Carlin, D., and Connor, E. (1998). Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group 17:110–115.
Sun, L. K., Fung, M. S., Sun, W. N., Sun, C. R., Chang, W. I., and Chang, T. W. (1995). Human IgA monoclonal antibodies specific for a major ragweed pollen antigen. Biotechnology (NY) 13:779–786.
Switzer, I. C., Loney, G. M., Yang, D. S., and Underdown, B. J. (1992). Binding of secretory component to protein 511, a pIgA mouse protein lacking 36 amino acid residues of the C alpha 3 domain. Mol. Immunol. 29:31–35.
Tamer, C. M., Lamm, M. E., Robinson, J. K., Piskurich, J. F., and Kaetzel, C. S. (1995). Comparative studies of transcytosis and assembly of secretory IgA in Madin-Darby canine kidney cells expressing human polymeric Ig receptor. J. Immunol. 155:707–714.
Taylor, A. K., and Wall, R. (1988). Selective removal of alpha heavy-chain glycosylation sites causes immunoglobulin A degradation and reduced secretion. Mol. Cell. Biol. 8:4197–4203.
Terskikh, A., Couty, S., Pelegrin, A., Hardman, N., Hunziker, W., and Mach, J. P. (1994). Dimeric recombinant IgA directed against carcino-embryonic antigen, a novel tool for carcinoma localization. Mol. Immunol. 31:1313–1319.
Tomana, M., Niedermeier, W., Mestecky, J., and Skvaril, F. (1976). The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry 13:325–328.
Trill, J. J., Shatzman, A. R., and Ganguly, S. (1995). Production of monoclonal antibodies in COS and CHO cells. Curr. Opin. Biotechnol. 6:553–560.
Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H., and Bailey, J. E. (1999). Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17:176–180.
van Egmond, M., van Vuuren, A. J., Morton, H. C., van Spriel, A. B., Shen, L., Hofhuis, F. M., Saito, T., Mayadas, T. N., Verbeek, J. S., and van de Winkel, J. G. (1999). Human immunoglobulin A receptor (Fc.RI, CD89) function in transgenic mice requires both FcR g chain and CR3 (CD11b/CD18). Blood 93:4387–4394.
van Ree, R., Cabanes-Macheteau, M., Akkerdaas, J., Milazzo, J. P., Loutelier-Bourhis, C., Rayon, C., Villalba, M., Koppelman, S., Aalberse, R., Rodriguez, R., Faye, L., and Lerouge, P. (2000). Beta(1, 2)-xylose and alpha(1, 3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 275:11, 451–11, 458.
Vidarsson, G., van Der Pol, W. L., van Den Elsen, J. M., Vile, H., Jansen, M., Duijs, J., Morton, H. C., Boel, E., Daha, M. R., Corthesy, B., and van De Winkel, J. G. (2001). Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166:6250–6256.
Weikert, S., Papac, D., Briggs, J., Cowfer, D., Tom, S., Gawlitzek, M., Lofgren, J., Mehta, S., Chisholm, V., Modi, N., et al. (1999). Engineering Chinese hamster ovary cells to maximize sialic acid content of recombinant glycoproteins. Nat. Biotechnol. 17:1116–1121.
White, K. D., and Capra, J. D. (2002). Targeting mucosal sites by polymeric immunoglobulin receptor-directed peptides. J. Exp. Med. 196:551–555.
Wolbank, S., Kunert, R., Stiegler, G., and Katinger, H. (2003). Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J. Virol. 77:4095–4103.
Yoo, E. M., Coloma, M. J., Trinh, K. R., Nguyen, T. Q., Vuong, L. U., Morrison, S. L., and Chintalacharuvu, K. R. (1999). Structural requirements for polymeric immunoglobulin assembly and association with J chain. J. Biol. Chem. 274:33, 771–777.
Zeitlin, L., Cone, R. A., and Whaley, K. J. (1999). Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg. Infect. Dis. 5:54–64.
Zhang, W., and Lachmann, P. J. (1994). Glycosylation of IgA is required for optimal activation of the alternative complement pathway by immune complexes. Immunology 81:137–141.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2007 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Yoo, E.M., Chintalacharuvu, K.R., Morrison, S.L. (2007). Recombinant IgA Antibodies. In: Kaetzel, C.S. (eds) Mucosal Immune Defense: Immunoglobulin A. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-72232-0_15
Download citation
DOI: https://doi.org/10.1007/978-0-387-72232-0_15
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-72231-3
Online ISBN: 978-0-387-72232-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)