Abstract
Although the structural diversity of sialic acid (Sia) is rapidly expanding, understanding of its biological significance has lagged behind. Advanced technologies to detect and probe diverse structures of Sia are absolutely necessary not only to understand further biological significance but also to pursue medicinal and industrial applications. Here we describe analytical methods for detection of Sia that have recently been developed or improved, with a special focus on 9-O-acetylated N-acetylneuraminic acid (Neu5,9Ac), N-glycolylneuraminic acid (Neu5Gc), deaminoneuraminic acid (Kdn), O-sulfated Sia (SiaS), and di-, oligo-, and polysialic acid (diSia/oligoSia/polySia) in glycoproteins and glycolipids. Much more attention has been paid to these Sia and sialoglycoconjugates during the last decade, in terms of regulation of the immune system, neural development and function, tumorigenesis, and aging.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Chemical analysis
- Deaminoneuraminic acid
- Disialic acid
- DMB derivatization
- Immunochemical analysis
- Immunohistochemistry
- N-Glycolylneuraminic acid
- O-Acetylated sialic acid
- Oligosialic acid
- O-Sulfated sialic acid
- Polysialic acid
1 Introduction
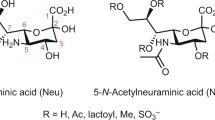
All cells are covered with glycoproteins and glycolipids, the biosynthesis of which takes place in the endoplasmic reticulum and Golgi compartments, and involves a variety of enzymes. The expression of some of these enzymes has been shown to be important in embryogenesis, cancer, pathogen recognition, and inflammation. N- and O-glycans and glycosphingolipids are often terminated with sialic acid (Sia), a family of nine carbon carboxylated monosaccharides, with high structural diversity. Sia consists of N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), deaminoneuraminic acid (Kdn), and their derivatives with modifications, such as acetylation, lactylation, methylation, and sulfation at the 4, 7, 8, and 9 positions (Fig. 1) [1–3]. These modifications of Sia have been implicated in embryonic development, protection from microbes and viruses, and modulation of complement activation [1, 2].
Sia exists as either free or bound sugar linked to galactose, N-acetylgalactosamine, and other sugar residues in various positions. Furthermore, Sia links to Sia itself to form dimeric, oligomeric, and polymeric structures (diSia/oligoSia/polySia). Thus, Sia shows extremely high structural diversity due to monosaccharide species as well as linkage modes. No other monosaccharide exhibits this structural diversity. Although there are some reports showing the biological significance of Neu5Gc and Neu5,9Ac [1, 4–6], for the majority of modified Sia, biological roles have not been well elucidated. For this purpose, specific methods or probes to detect specifically modified Sia are absolutely necessary. In this chapter we summarize current chemical and immunochemical methods to detect modified Sia species, with a special focus on O-acetylated Neu5Ac, Neu5Gc, Kdn, O-sulfated Sia, as well as diSia, oligoSia, and polySia. Because other chapters in this volume specifically focus on O-acetylated Neu5Ac [7], Neu5Gc [8], and polySia [9], we restrict ourselves in this chapter to a brief introduction of the different Sia species.
1.1 O-Acetylated Sialic Acids (SiaAc)
The O-acetylated Sia (SiaAc) at the 4, 7, 8, and 9 positions frequently occurs in glycoproteins and glycolipids. SiaAc is expressed in cell type-specific and developmental stage-specific manners, and is involved in various biological phenomena, such as immune reactions, virus-cell adhesion, malignancy, and others [1, 10]. Sialate:O-acetyl-transferases [1, 11] and sialate:O-acetyl-esterases [12–14] are important enzymes for acetylation and deacetylation of Sia. The importance of 9-O-acetylated Sia during early development was evidenced by the transgenic expression of Influenza C hemagglutinin esterase in mice, leading to mice deficient in 9-O-acetylated Sia [4]. Recent analyses on sialate:O-acetyl-esterase deficient mice showed impairment of B cell-related functions that could be related to the inhibitory activity of siglec-2 ([5]; siglecs are discussed by Schwardt et al. [15]).
1.2 N-Glycolylneuraminic Acid (Neu5Gc)
Neu5Gc is broadly expressed in mammals. Neu5Gc is synthesized by the CMP-Neu5Ac hydroxylase (CMAH), a converting enzyme of CMP-Neu5Ac to CMP-Neu5Gc [16, 17]. Due to a genetic defect in CMAH, humans do not express a functional CMAH and thus do not endogenously produce Neu5Gc. However, Neu5Gc can be metabolically incorporated from dietary sources (particularly red meat and milk) into glycoproteins and glycolipids. Human fetuses, tumors, and some normal tissues were shown by mass spectrometry to incorporate Neu5Gc. Moreover, Neu5Gc has been demonstrated to be metabolically incorporated and covalently expressed on cultured human cell surfaces. Metabolically incorporated Neu5Gc is immunogenic in humans [18], and the presence of human anti-Neu5Gc antibodies has been associated with tumor progression [19] and vascular inflammation [20]. For additional details see Shilova et al. [21] and Davies and Varki [8].
1.3 Deaminoneuraminic Acid (Kdn)
Kdn contains a hydroxyl group at the C-5 position, instead of the acetamide-group in Neu5Ac (Fig. 1). Kdn was discovered in glycoproteins from rainbow trout eggs [22, 23]. In mammalian tissues, Kdn occurs at a very low level and amounts to <1% of total Sia. However, expression of Kdn increases in certain ovarian cancers, and the amount is proportional to the degree of malignancy [24]. Under hypoxic conditions free Kdn increases in mammalian tumor cells. As hypoxia-resistance is a frequent property found in tumors, it is likely that Kdn supports this state of mammalian cells [25]. In rat liver, cytosolic Kdn increases in an age-dependent manner. The level of free Kdn was reported to be high in erythrocytes of the umbilical vein. These findings demonstrate that attention has to be paid to the physiological significance of Kdn in malignancy and in aging [23]. Of interest in this context, Kdn residues, in contrast to Neu5Ac and Neu5Gc, are resistant to known bacterial and mammalian sialidases [22, 23]. In salmonid fish, Kdn caps polySia chains in egg polysialoglycoproteins, thus stopping chain elongation and possibly protecting polySia from bacterial sialidase attacks [22, 23]. Biological roles of Kdn in mammals remain to be elucidated, but increase of the Kdn level in cells and tissues may be a biomarker for some cancers and in aging cells.
1.4 O-Sulfated Sialic Acids (SiaS)
Sias are sometimes esterified with sulfate group to give O-sulfated Sia (SiaS). Methods for the detection of SiaS are not so well developed, and occurrence of SiaS has been only demonstrated in a limited number of animal species so far [26–34]. In sea urchin, 8-O-sulfated Neu5Ac (Neu5Ac8S) and Neu5Gc8S are present in glycolipids [27–29], in glycoproteins from the vitelline layer polysialylated glycoprotein [30], and in the sperm flagellar glycoprotein, flagellasialin [31, 32]. In mammals, Neu5Ac8S and Neu5Gc8S residues are found in gangliosides from bovine gastric mucosa [33, 34], and Neu5Ac8S was detected in tissues from human, rat, and mouse [35, 36]. The glycoconjugates carrying SiaS in mammals have remained undetermined with the exception of the bovine gastric gangliosides [33, 34]. Similarly, the biological significance of the SiaS in animal tissues is not known. It is interesting, however, that chemically synthesized SiaS-containing compounds have been applied as artificial inhibitors for fertilization [37] and bacterial infections [38], irrespective of the very limited knowledge on the natural occurrence of SiaS. These facts point to the potential importance of the SiaS residues in various biologic processes.
1.5 Di-, Oligo-, and Polysialic Acids (diSia/oligoSia/polySia)
In most cases, Sias are present as α2,3- or α2,6-linked monosialyl residues at the non-reducing terminal positions of glycan chains on glycoproteins and glycolipids. In two cases Sia has been identified as an internal sugar between neutral sugars: Fucα1→O glycolyl Neu5Gcα2-4Neu5Acα2-6Glc1- in echinoderms [39] and a polymer of →4Neu5Acα2→6Glc/Gal1 disaccharide in the capsular polysaccharides of Neisseria meningitidis serogroups Y and W-135, respectively [40]. Moreover, Sias can be linked to each other to form diSia, oligoSia, and polySia (by definition DP > 8 is termed polySia). First identified in the glycocalyx of neuroinvasive bacteria, polySia has so far been demonstrated to be a posttranslational modification on six glycoproteins, the fish egg polysialoglycoprotein (PSGP), the neural cell adhesion molecule (NCAM), a voltage-gated sodium channel in eel, CD36 in human milk, NRP2 in human lymphocytes, and SynCAM-1 in mouse brain (for recent reviews see [41–43]). Polysialylation occurs in some tumors (probably on NCAM) and is involved in metastasis. By virtue of its net negative charge at physiological pH and exclusive volume, polySia serves as a mediator of ligand–receptor and cell–cell interactions via an anti-adhesive effect [44]. In addition, polySia has been demonstrated to function as a reservoir molecule for BDNF, dopamine, and FGF-2 [41, 43, 45–49] and to be involved in the regulation of ion transport via interactions with channels [32, 50–52]. Compared to what is known about polySia, the information on oligoSia is limited [43, 53, 54]. DiSia and oligoSia are common glyco-epitopes between glycolipids and glycoproteins. The functions of diSia on the glycolipids (GD3 and GT1b) have been well studied, while far less knowledge about the functions of diSia and oligoSia on the glycoproteins has been reported [55–57]. Interestingly, oligoSia and polySia with the degree of polymerization up to 16 have been recently found in glycolipids of sea urchin sperm [58], although their functions remain to be elucidated.
2 Chemical Analyses of Sialic Acid and Sialoglycoconjugates
2.1 Detection of Modified Sialic Acids
2.1.1 Colorimetric Analyses
To quantitate the amount of Sia at 0.1–100 μg, colorimetric analyses are most common with simple methods, and include the thiobarbituric acid [59, 60] and resorcinol methods [61]. In the resorcinol method, Neu5Ac and Neu5Gc in either free or bound form can be equally detected, while Kdn gives no color. In the thiobarbituric acid, Neu5Ac and Neu5Gc are detected only in free form, while Kdn can be detected in both free and bound forms. To quantitate the amount of Sia below 0.1 μg, a highly sensitive fluorometric high-performance liquid chromatography (HPLC)-based method is usually used. Here we focus on the detailed description of fluorometric HPLC technology.
2.1.2 Fluorometric HPLC Analysis
Fluorometric HPLC analysis is currently the most sensitive and reliable method for quantitative detection of Sia in the pmol range. In this analysis, a free form of various Sia species is labeled with the fluorescent dye 1,2-diamino-4,5-methylenedioxybenzene (DMB), and fluorometrically analyzed on HPLC [62, 63]. DMB is a reagent which specifically reacts with α-keto acids, and detects not only Sia but also typical α-keto acids such as pyruvate and α-ketoglutarate. Fluorometric HPLC analysis consists of the following steps: step 1, acid hydrolysis of sialoglycoconjugates to release free Sia; step 2, fluorescent labeling of released Sia with DMB; step 3, separation and quantification of DMB-labeled Sia on HPLC.
Fluorometric HPLC analysis involves two acidic conditions. One is a hydrolysis, 0.1 N trifluoroacetic acid at 80°C for 2 h to release free Sia from sialoglycoconjugates at step 1. The second is the DMB labeling which takes place in 0.01 N trifluoroacetic acid at 50°C for 3 h (step 2). Attention must therefore be given to the acid lability of some Sia substituents. No significant degradation of Neu5Ac, Neu5Gc, and Kdn has been reported. For SiaS, the glycosidic bonds of SiaS were completely hydrolyzed under the hydrolysis conditions, while their sulfate esters are hydrolyzed by, at most, 4% [64]. No apparent desulfation occurred under the labeling conditions. The O-acetyl groups on Sia are very labile to basic conditions and more stable under acidic conditions. Although no quantitative data are available for the lability of O-acetyl group during the DMB-derivatization procedures, quantitative analysis is basically possible as long as authentic O-acetylated Sia compounds are used as a standard. It is of interest to note that the 8-O-acetyl ester is more labile than a 9-O-acetyl ester, and acetyl-groups tend to migrate to the C-9 position at the end, even under neutral conditions at room temperature [65]. However, no such migration takes place with the 8-O-sulfate ester of Sia.
In Step 3, DMB derivatives of Neu5Ac, Neu5Gc, and Kdn are separately quantitated by HPLC on an octadecylsilyl (ODS) column using acetonitrile/methanol/water (9:7:84 by volume) as the elution solution [24]. The O-acetylated Sia are eluted more slowly than the corresponding non-O-acetylated Sia. To separate the various SiaS from each other, an acidic solution of acetonitrile/methanol/0.05% trifluoroacetic acid (9:7:84 by volume) [64] is recommended. This solution will enable the separation of DMB derivatives of Neu5Ac8S, Neu5Ac9S, Neu5Gc8S, and Neu5Gc9S. For the separation of DMB derivatives of Kdn8S and Kdn9S, a solution with higher polarity, acetonitrile/methanol/0.05% trifluoroacetic acid (4:6:90 by volume), is appropriate [64]. The described protocol enabled the separation of DMB derivatives of nine different sulfated and non-sulfated Neu5Ac, Neu5Gc, and Kdn (Fig. 2). The relative peak area represents the proportion of the peak area of each DMB-Sia to that of DMB-Neu5Ac, and can be used as an index for relative detection efficiency. Typical relative peak areas to that of DMB-Neu5Ac are shown in Table 1.
Fluorometric HPLC of sialic acid. DMB-derivatives of Neu5Ac, Neu5Gc, Kdn, and their 8- or 9-O-sulfated forms are shown. DMB-derivatives were applied to an ODS column, eluted with acetonitrile/methanol/0.05% trifluoroacetic acid (4: 6: 90 by volume), and detected fluorometrically at excitation/emission 373/448 nm. The retention times (min) for the DMB-derivatives are: 13.9, DMB-Kdn9S (1); 14.4, DMB-Kdn8S (2); 16.3, DMB-Neu5Gc9S (3); 17.2, DMB-Neu5Gc8S (4); 21.8, DMB-Neu5Ac9S (5); 22.8, DMB-Kdn (6); 23.4, DMB-Neu5Ac8S (7); 27.9, DMB-Neu5Gc (8); 40.0, DMB-Neu5Ac (9)
2.2 Detection of Oligo/Polysialic Acids
When samples containing diSia, oligoSia, and polySia structures at 10–100 μg are analyzed, conventional methods including methylation analysis [66], NMR (nuclear magnetic resonance) [67], and mild acid hydrolysis-TLC (thin layer chromatography) [68] can be applied. However, the amount of these types of glycoproteins is often too small to be analyzed by conventional methods. The fact that the di-, oligo-, and polySia-modification of glycoproteins has not often been reported suggests that these species in an organism is rare. As highly sensitive chemical methods to analyze minute amounts of di-, oligo-, and polySia were developed using highly sensitive fluorescent reagents [62, 69–72], the number of studies identifying di-, oligo-, and polySia increased gradually [43]. Therefore, in this chapter we are focusing on the chemical and immunochemical detection of small amounts (picogram to nanogram amounts) of sialic acid which may serve as powerful tools to reveal the importance of di-, oligo-, and polySia in nature.
The polySia glycotope exhibits structural diversity in the sialic acid components (Neu5Ac, Neu5Gc, and Kdn) in the inter-sialyl linkages (α2→5O glycolyl , α2→8, α2→9, and α2→8/9) and in the degree of polymerization (DP) [43, 68]. With a highly specific reagent for α-keto acid [62], a specific labeling of di-, oligo-, and polySia became possible and products could be detected by anion exchange chromatography. This broadly used method was first developed by us [70] and was further improved in later studies [73]. With this method, di-, oligo-, and polySia on glycoproteins were frequently demonstrated [43, 53, 54].
2.2.1 Mild Acid Hydrolysis-Fluorometric HPLC Analysis
Gangliosides, glycoproteins in solution or blotted on polyvinylidene difluoride (PVDF) membrane (Immobilon P, Millipore, U.S.A) are hydrolyzed with 0.01 N trifluoroacetic acid (TFA) at 50°C for 1 h to release di-, oligo-, and polySia units from the glycoconjugates. The samples were then freeze dried. To release di-, oligo-, and polySia, 20 μL of 0.01 N TFA and 20 μL of 7 mM DMB solution in 5.0 mM TFA containing 1 M 2-mercaptoethanol and 18 mM sodium hydrosulfite, are added to the samples These samples are incubated at 50°C for 1–2 h or at 4°C overnight. The DMB-labeled samples are applied to an HPLC analysis. HPLC equipped with a Mono or Mini Q HR5/5 (0.5 × 5 cm, GE, Uppsala, Sweden), Resource Q (1 mL, GE, Uppsala, Sweden), CarbopacPA-100, or DNApak1 PA-120 (4 × 250 mm, Dionex) anion exchange column, and a fluorescence detector (FP-2025, JASCO). After equilibrating the column with 5 mM to 20 mM Tris-HCl (pH 8.0) at 26°C, samples are applied and the DMB-labeled di-, oligo-, and polySia are eluted at a flow rate of 0.5–1.0 mL/min with a linear gradient of NaCl (0→0.4 M) after 15–30 min wash with 5–20 mM Tris-HCl (pH 8.0) (flow through). The fluorescence of the DMB-labeled samples is detected with a fluorescence detector at excitation 373 nm and emission 448 nm. The separation is dependent on the anion exchange column and buffer conditions. Mono Q and Resource Q are suitable for shorter oligo- and polymers. Mini Q and Carbopac PA-100 have almost the same ability to separate polymers with DPs ranging from 2 to DP 50–90 (Fig. 3). Additionally, this method can be applied to determine the glycosidic linkage (α2→8, α2→9, and α2→5) of diSia units (Neu5Ac→Neu5Ac, Neu5Ac→Neu5Gc, Neu5Gc→Neu5Ac, Neu5Gc→Neu5Gc, Kdn→Kdn, and so on) and their component Sia species [70] according to their elution time. If the sample is pure sialyloligo or -polymer, it can be detected on a UV detector or a pulse amperometric detector (PAD) [71], instead of the DMB-derivatization/fluorometric detection method.
Mild acid hydrolysis-fluorometric HPLC analysis. Mini Q anion exchange chromatography of α2→8-linked di/oligo/polyNeu5Ac-DMB. α2→8-Linked oligo/polyNeu5Ac was labeled with DMB and applied to a mini Q HR5/5 anion exchange column (1 mL, Cl−-form). The column was eluted with 5 mM Tris-HCl (pH 8.0) with a gradient from 0 to 0.3 M NaCl for 75 min and 0.3 M NaCl to 0.4 M NaCl for 120 min after 15 min wash. The elution was monitored by a fluorescence detector (set at wavelength of 373 nm excitation and 448 nm emission). Each peak is assigned from the order of elution, based on DP
To increase the sensitivity, especially in higher DP, because the labeling site is one per chain, it is recommended to separate the polySia on an anion exchange column and to label each fraction with DMB labeling after hydrolysis of the sample with 0.1 N TFA at 80°C for 1–2 h [72]. The sensitivity increases depending on their DP.
2.2.2 Fluorometric C7/C9 Analysis
The periodate treatment of sialic acid residing in non-reducing terminal ends is a well-known method to modify the terminal sialic acid [74]. To observe and quantify the internal sialic acids of α2→8-linked di-, oligo-, and polySia-containing glycoconjugates, sensitive chemical methods were developed with highly sensitive fluorescent labeling reagents (DMB) as described above [69].
To perform the fluorometric C7/C9 analysis, several reagents are prepared. Solutions A–G are prepared as follows: solution A, 40 mM sodium acetate buffer (pH 5.5); solution B, 0.25 M periodate; solution C, 3% ethyleneglycol; solution D, 0.2 M sodium borohydride in 0.2 M sodium borate buffer (pH 8.0); solution E, 0.2 M trifluoroacetic acid (TFA); solution F, 0.01 M trifluoroacetic acid; solution G, 7 mM 1,2-diamino-4,5-methylenedioxybenzene (DMB) in 5 mM trifluoroacetic acid containing 1 M 2-mercaptoethanol and 18 mM sodium hydrosulfite. For glycoproteins or oligosaccharides in solution, samples (0.25–1,000 ng as Sia) are dissolved in 25 μL of solution A and 2 μL of solution B are added. After being left at 0°C for 3 h in the dark, 5 μL of solution C and 32 μL of solution D are added successively and allowed to stand at 0°C overnight. To the resultant mixture is added a pre-determined amount of Kdo (for example, as an internal standard for quantitating the resultant sialic acids) and the volume is set to 100 μL with water. Following further addition of 100 μL of solution E to adjust to 0.1 M TFA, the mixture is hydrolyzed at 80°C for 2–4 h. The hydrolysate is lyophilized by a Speed Vac. After addition of 20 μL of solution F to the samples, 20 μL of solution G is added and the samples are incubated at 50°C for 2 h (it depends on the DP). The resulting supernatants are applied to an HPLC for analysis. This method can also be applied to the samples blotted on the PVDF membrane.
The DMB-labeled sialic acids are applied to the HPLC equipped with a TSK-gel ODS-120T column (250 × 4.6 mm i.d., Tosoh), and a fluorescence detector (FP-2025, JASCO). The column is equilibrated using acetonitrile/methanol/water (9:7:84 by volume) at 26°C. Then 2–20 μL of the supernatants are applied to HPLC analysis for an isocratically flow at 1.0 mL/min and the DMB-labeled sialic acid is detected with a fluorescence detector at an excitation of 373 nm and an emission at 448 nm (Fig. 4). The estimate of the ratios of the quantity of internal sialic acid residues (C9(Sia)) to that of total sialic acid residues (C7(Sia) + C9(Sia)) can then be obtained.
HPLC profile in the fluorometric C7/C9 analysis. A typical elution profile of DMB derivatives of C7-analogues and authentic sialic acids (C9) on the fluorometric HPLC. Disialyl silalitols, 12.5 ng each of Neu5Acα2→8Neu5Acα2→8-Neu5Ac-ol and Neu5Gcα2→8Neu5Gcα2→8-Neu5Gc-ol, were subjected to the periodate oxidation/reduction/hydrolysis, DMB derivatization, and fluorometric HPLC on a TSK-gel ODS-120T column (250 × 4.6 mm i.d.). The column was eluted with acetonitrile/methanol/water (9:7:84 by volume) at 1.0 mL/min at 26°C. Elution profile was monitored by measurement of fluorescence: excitation, 373 nm; emission, 448 nm
The following limitations should be noted with respect to this method. First, this method is applicable to only α2→8-linked oligo/polymer of N-acylneuramininic acid, and cannot be used for DP analyses of α2→9, α2→8/α2→9-mixed linkage polymers, or α2→5O glycolyl -linkage. Second, the C9-derivatives formed do not always arise from α2→8-linkages, because 8-O-substituted Neu5Acyl residues may also give the same C9-derivatives. Therefore, mild alkali treatment of samples is usually carried out prior to periodate oxidation. Third, the molar proportion of C9-derivatives to C7-derivatives does not directly represent the DP unless it is a linear polySia chain. Thus, the method does not yield the DP for multiply sialylated chains present in the same sample. In general, glycoproteins have more than one glycan chain and even one glycan chain bears two to four non-reducing terminal residues that may be terminated by monoSia or oligoSia residues.
3 Immunochemical Analyses of Sialic Acid and Sialoglycoconjugates
3.1 Immunochemical Detection of Kdn and O-Sulfated Sialic Acids
3.1.1 Immunodetection of Kdn Using Kdn-Specific Antibodies and Kdn’ase Sm
Kdn residues are resistant to various bacterial sialidases that are often used as reagents. However, a bacterial sialidase from Sphingobacterium multivorum, named Kdn’ase Sm, has a unique property in that it cannot cleave the glycosidic linkages of Neu5Ac and Neu5Gc in any free glycans and glycoconjugates but can specifically cleave the Kdn glycosidic linkages in free glycans, glycolipids and glycoproteins [75]. Similar Kdn’ases are also known in hepatopancreas of oyster. These enzymes strictly discriminate Kdn from Neu5Ac or Neu5Gc, and thus provide evidence for Kdn-specific recognition phenomena in these organisms.
Two monoclonal antibodies recognizing Kdn-containing epitopes have been developed. Kdn8Kdn, a mouse IgM, recognizes α2,8-linked oligo/polyKdn with more than two residues [76]. mAb.kdn3G, a mouse IgG, recognizes the Kdnα2,3Gal- structure [77]. In immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and Western blotting using these antibodies, the results would be more reliable, if the loss of antigenicity by digestion with Kdn’ase Sm, a bacterial Kdn-specific sialidase (see below), was confirmed. Of the known lectins that recognize Neu5Ac, Sambucus sieboldiana lectin (SSA) can recognize the Kdnα2,6Gal-linkage better than Neu5Acα2,6Gal-linkage [78]. In a combination of SSA and Kdn’ase Sm, Kdnα2,6Gal-structure can be detected.
Kdn can be detected in free glycans, glycolipids, and glycoproteins in various cells and tissues from animals that express Neu5Ac. The expression level is very low in mammals.
3.1.2 Immunodetection of SiaS Using Anti-SiaS Antibodies
Two monoclonal antibodies, mAb.3G9 and mAb.2C4, specifically recognizing SiaS, have been reported. The mAb.3G9 is a mouse IgM, and is highly specific for Neu5Ac8S. The antibody was generated using sea urchin sperm as an immunogen [79]. The mAb.2C4 is also a mouse IgM and recognizes Neu5Ac8S and Neu5Gc8S, which can compensate for the binding specificity of mAb.3G9. This antibody was prepared using sea urchin egg low density detergent-insoluble membrane as an immunogen [64]. Detection limits of mAb.3G9 (1 μg/mL) and mAb.2C4 (culture supernatant) for Neu5Ac8Sα2→8Neu5Acα2→6Glc-Cer in ELISA were 0.94 and 1.9 pg/well, respectively [64]. As in the case with Kdn’ase Sm in immunodetection of Kdn, an enzyme specifically destroying the SiaS epitopes would be a powerful tool for reliable detection. Commercially available arylsulfatases, which can remove sulfate group from sulfatide containing 3-O-sulfated Gal residue, have no activities on sulfate group on Sia. There are no known sulfatases that can act on the sulfate ester on SiaS. Therefore, immunochemical detection should be confirmed at least by chemical evidence to obtain reliable conclusions of the localization of SiaS.
Western blotting of sperm lysate of Hemicentrotus pulcherrimus using mAb.3G9 detects unique smear at 40–80 kDa, which corresponds to flagellasialin containing Neu5Ac8S-capped α2,9-Neu5Ac-linked polymers [31, 32].
3.2 Immunochemical Detection of Oligo- and Polysialic Acids by Immunoblotting and ELISA
3.2.1 Immunodetection of Antibodies Recognizing di/oligo/polySia
Antibodies are powerful tools for studying the structure and function of glycotopes. However, it is very important that the immunospecificities toward linkage, DP, and component are determined in detail before use.
For α2→8-linked polySia glycotopes, several antibodies have been developed and used for the past 2 decades. Among the “so-called anti-polySia antibodies,” the horse polyclonal antibodies H.46 [80] and mouse mAb 735 [81] had been the only two whose immunospecificities were specifically determined by inhibition assays [82, 83]. The immunospecificity of the majority of other “anti-polySia antibodies” remained unknown until a comprehensive examination of the immunospecificity of these “anti-polySia” antibodies was carried our using an ELISA-based method with phosphatidylethanolamine-conjugated oligo- and polySia chains as antigens [84]. It was demonstrated that the “anti-polySia antibodies” discriminate in terms of the species of Sia residues and chain length. Our group has also developed a new anti-diSia antibody using copolymers of α2→8-linked N-acetylneuraminyl p-vinylbenzylamide and acrylamide as an immunogen [85]. Thus, a large number of antibodies recognizing di-, oligo-, and/or polySia structures with defined specificities now exist and are summarized in Table 2.
Interestingly, the anti-oligo/polySia antibodies can be classified into three groups, based on the immunospecificity for chain length and the involvement of the non-reducing terminus in the antibody recognition site. Group I consists of the “anti-polySia antibodies” that recognize chains of α2→8-linked Sia with a DP ≥ 8, including fully extended polySia chains with a DP ~ 200–400. These antibodies recognize the helical conformation formed by Sia residues within the internal region of the polySia chains, but not the non-reducing terminal residues. Group II antibodies, designated as “anti-oligo + polySia antibodies,” recognize both oligoSia with DP 2–7 and polySia chains. These antibodies recognize the distal portion of oligo/polySia chains, including the non-reducing termini. Group III antibodies, designated as “anti-oligoSia antibodies,” recognize specific conformations of di- and oligoSia with a DP 2–4, but do not bind polySia. Group III antibodies can be further classified into two subgroups. One group is composed of “anti-diSia antibodies” that recognize the diNeu5Ac structure (S2-566, 1E6), and the other includes the “anti-oligoSia antibodies” (AC1). Group II and III antibodies would be useful for detecting and determining di- and oligoSia structures, in combination with treatment with exo- and endo-sialidases, as described below (Table 3). The antibodies can be applied to Western blotting (Fig. 5a), immunocytochemistry (Fig. 5b), and FACS analysis (Fig. 5c) [86].
TriNeu5Ac epitopes in mouse brain homogenates and Neuro2A cells. (a) Western blots of mouse brain homogenates with anti-trSia antibody (A2B5) and anti-oligo/polySia antibody (12E3). The photos are cited from [85]. (b) Cell surface staining with A2B5 using fluorescent microscopy. The mouse neuroblastoma cells were incubated with retinoic acid at 20 μM in 2% FBS in DMEM and differentiated into neurons. At day 3, the cell surfaces were immunostained with anti-triSia antibody (A2B5, Green), and anti-NCAM antibody (Red). (c) Cell surface immunostaining with anti-triSia antibody using flowcytometer. Neuro2A cells were transfected with ST8Sia III gene although the cells have its enzyme endogenously. The stably ST8SiaIII expressing cells were immunostained with anti-triSia antibody before, and after exosialidase treatment
Surprisingly, anti-diSia antibodies obtained after immunization with gangliosides, developed, and used as anti-ganglioside antibodies can recognize cytosolic proteins such as carbonic anhydrase II [87] although they recognize diSia epitope on glycoproteins. These phenomena might explain the cytosolic immunostaining with those anti-ganglioside antibodies in part. The protein mimicry should be considered and confirm the epitope with those antibodies.
3.2.2 Use of Endo-Sialidases and Exo-Sialidases for the Detection of OligoSia and PolySia
Endo-sialidase (for a detailed description see Jakobsson et al. [88]) can serve as a specific molecular probe to detect and selectively modify α2→8-linked polySia chains. The soluble enzyme derived from bacteriophage K1F, designated endoN, catalyzes the depolymerization of polySia chains as follows: (→8Neu5Acylα2→) n −X (n ≥ 5) → (→8Neu5Acylα2→)2−4 + (→8Neu5Acylα2→)2−X. Two other types of endo-sialidases whose substrate specificities are different from the endoN of bacteriophage K1F [89] have been isolated: endoNE [90] and an endosialidase [91] from a bacteriophage. The minimum chain length required for cleavage is DP ≥ 11 and DP ≥ 3, respectively. Exo-sialidases have been isolated that cleave specific linkages, for example, α2,3-sialidase (NANase I), α2,3-, α2,6-sialidase (NANase II), α2,3-, α2,6-, α2,8-sialidase (NANase III), and α2,3-, α2,6-, α2,8-, and α2,9-sialidase. As the endoN is insensitive toward di- and oligoSia structures and they should be cleavable by these exosialidases, it is possible to confirm the length of given di-, oligo-, and polySia chains in conjunction with endo- and exo-sialidase treatments before and after immunostaining with anti-diSia, oligoSia, and polySia antibodies (See Table 3) [43, 53, 54].
Finne et al. established a specific probe for detection of polySia from endoNE that inactivates its enzymatic activity but not its ability to bind to polySia [92]. They succeeded in the detection of polySia-NCAM with this probe.
4 Immunohistochemistry for Specific Sialic Acids
When tissues are removed from the body during surgery, or during an autopsy, the onset of autolysis occurs promptly. This can thwart efforts to isolate nucleotides or certain enzymes and proteins, or to perform high quality histology. Thus tissue ideally has to be flash-frozen for extracts, or frozen in cryoprotective agents, or fixed, using different procedure-dependent fixatives, for analysis using histopathology. Immunohistochemistry is the process whereby tissue sections are probed with antibodies to determine which cells contain epitopes of interest. The bound antibody is then detected using secondary and/or tertiary reagents, using fluorescent tags, or enzyme labels. If using enzyme labels, detection is facilitated using a number of different substrates, which produce precipitates, which are visible under the microscope. The nuclei are counterstained so that the morphology of the tissue can then be recognized during the analysis of the slides. In all immunohistochemistry experiments, to determine specificity, it is important to use specific blocking or inhibitory reagents. Processing of tissues into paraffin destroys many antigenic epitopes and additional unmasking methods often have to be used to detect antigens that are left after the processing. However, some changes are irreversible, such as the extraction of glycolipids during the paraffin embedding process. Immunohistochemistry remains an exact science, and appropriate use of controls and blocking agents are critical while analyzing results.
Lectins are carbohydrate-binding proteins, usually derived from plants, and have been used as tools to detect changes in glycan branching presentation on different cells. Mice that have been genetically altered to be deficient for specific sialyltransferases are then useful as important controls to demonstrate specificity of some lectin binding patterns. This was demonstrated nicely in a report [93] describing mice deficient in the sialyltransferases ST6Gal-I (transfers α2-6-linked Sia to Galβ1-4GlcNAc units, mostly on N-glycans), or ST3Gal-I (transfers α2-3-linked Sia to Galβ1-3GalNAc units, mostly on O-glycans). Sambucus nigra agglutinin (SNA) staining was lost in essentially all tissues of adult ST6Gal-I null mice, indicating that this is the only enzyme generating the Siaα2-6Galβ1-4GlcNAc sequence. Lectin histochemistry with the α2-3-Sia-specific Maackia amurensis Hemagglutinin (MAH or MAL-II) showed loss of binding in almost all tissues of ST3Gal-I null mice. However, use of the other isolectins of the Maackia amurensis seeds, the MAL-I lectin, or the MAA lectin, showed continued binding to many tissues from the ST3Gal-I null animals (Fig. 6).
Differences in binding to frozen sections of mouse thymus, from wild type animal (left column) and from ST3Gal-I homozygous mutant (right column) observed with MAL-I and MAL-II, detected using the Cy3 fluorescent streptavidin label. Top row shows hematoxylin and eosin stained sections of the thymus, with dark blue cortex and lighter staining medulla. Middle row shows binding of MAL-I to thymus from both wild type (left) and ST3Gal-I null animals (right). Bottom row shows binding of MAL-II (MAH) lectin to the thymic medulla of the wild type mouse (left) and only minimally to structures in the thymic medulla from ST3Gal-I null mouse (right). Scale bar = 500 μm
Different isolectins have been isolated from Maackia amurensis seeds [94, 95] and have been used as probes for sialic acids that are α2-3-linked to the penultimate galactose residues. The two most commonly used isolectins are the leukoagglutinin (MAL) and the hemagglutinin (MAH). Some commercial vendors use the term MAA to denote probes for sialic acids that are α2-3-linked, but in fact MAA is a mixture of MAL and MAH [96, 97]. In both MAL and MAH the glycine and asparagines involved in sugar binding were substituted by lysine and aspartic acid, respectively, [94, 98] and shown to be important in sialic acid binding. Investigators, using biochemical methods, have described the carbohydrate binding specificity of these lectins, and some have also described the lectin histochemistry profile. However, many of the histochemistry studies use MAL or MAA, and only rarely has MAH been used to ascribe the binding being used to identify α2-3-linked sialic acids. Most recently, it was shown that effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities [99].
4.1 Detection of 9-O-Acetylated Sialic Acids in Mammalian Tissues
In mammalian tissues, 9-O-acetylation is often associated with gut mucins, neural gangliosides, and erythrocytes. Direct demonstration of the presence of 9-O-acetylated Sias in mammalian tissues was indirect (modifications of the PAS technique) until the development of a chimeric dual-functional probe derived from influenza-C hemagglutinin esterase (InfCHE). This probe was constructed using a soluble and versatile form of the InfCHE that retains both the hemagglutinin and esterase activity and also has the binding properties of IgG Fc. The esterase activity is very similar to that of the native protein, and pH conditions can be adjusted to remove either 9-O-acetyl groups alone or both 9- and 7-O-acetyl groups completely (by causing migration of the latter to the 9-carbon position). DFP (di-isopropyl fluorophosphates) inactivation stabilizes and “unmasks” the hemagglutinin activity, resulting in a probe that specifically detects 9-O-acetylated sialic acids at ambient temperatures. Since CHE-FcD and CHE-Fc differ only by a single di-isopropyl group, each can be used as control for the other. Thus, when the CHE-Fc is used to remove 9-O-acetyl esters, the CHE-FcD can be used as an inactive control. Conversely, when CHE-FcD is used to probe for 9-O-acetylated Sias, the CHE-Fc can be used as a control for nonspecific binding. The two forms can even be used sequentially; i.e., treatment with CHE-Fc can be used to remove the 9-O-acetyl esters prior to probing with CHE-FcD, leaving only nonspecific staining, if any. It should be noted that the CHE-Fc does have a masked hemagglutinin activity, which can give a weak positive response at low temperatures, and even at ambient temperatures if the density of O-acetylated Sias is very high [6, 100–105].
Tissue expression of 9-O-acetylated Sias was best analyzed using frozen sections, using the probe CHE-FcD pre-complexed to the secondary antibody. There were differences in expression of 9-O-acetylated Sias in tissues isolated from rats and those from mice. As shown Fig. 7, there was abundant expression in rat liver, rat lung, in the glomeruli of rat kidney, and gray matter of rat brain. However, although mouse red blood cells and blood vessels showed expression of 9-O-acetylated Sias with the CHE-FcD probe, expression was not robust in mouse liver and mouse lung, but was instead present in the T-cell areas of lymphoid follicles in spleen, in the mature lymphoid medulla of the thymus, gray matter of the brain, and adrenal medulla.
Differences in binding seen with the probe for 9-O-acetylated sialic acids, CHE-FcD, to mouse and to rat sections. As shown, 9-O-acetylated sialic acids are abundantly expressed in rat liver but not in mouse liver, although it is present on mouse red blood cells (right). It is also expressed on rat kidney glomeruli but not in the mouse kidney where it is present only on some medullary vessels. There is a different distribution in spleen from rat and mouse, and is present in rat lungs but not in mouse lungs; and diffusely in rat brain (left) and only in gray matter of mouse brain (right). Scale bar = 50 μm
Since the submaxillary gland mucins of many species have been reported to have high concentrations of 9-O-acetylated Sias, it was surprising to find no significant staining in the parenchyma of the rat or mouse submaxillary gland. Lipid overlays and protein blots confirmed that this is not due to inaccessibility of molecules on the tissue section. It is possible that the sialic acids of rat submaxillary mucins are 4-O- rather than 9-O-acetylated [106].
The hemagglutinin esterases of influenza C viruses and certain nidoviruses, including group 2 coronaviruses, recognize 9-O-acetylated Sia-containing glycoconjugates on the surface of host cells, and some can remove the 9-O-acetyl moieties [5, 107, 108]. Recombinant soluble forms of the bovine coronavirus HE with and without inactivation of the esterase active site have been reported, and these molecules may be more stable than the InfCHE reagents. However, localization to tissue sections using immunohistochemistry has not yet been reported with the bovine coronaviruses reagents.
4.2 Detection of Neu5Gc in Human Tissues
Humans are genetically defective in synthesizing the common mammalian sialic acid Neu5Gc. It has been shown that Neu5Gc can be metabolically incorporated and covalently expressed on cultured human cell surfaces. Thus humans can also metabolically incorporate Neu5Gc into glycoproteins and glycolipids of human tumors, fetuses, and some normal tissues, likely from dietary sources (particularly red meats). Mass spectrometry confirmed the presence of small amounts of Neu5Gc in these human tissues.
As birds also do not synthesize Neu5Gc [109], it is possible to raise good anti-Neu5Gc antibodies in chickens, A polyclonal chicken anti-Neu5Gc was purified using a novel affinity method, utilizing sequential columns of immobilized human and chimpanzee serum sialo-glycoproteins, followed by specific elution from the latter column by free Neu5Gc, This purified anti-Neu5Gc antibody was used to characterize the expression of Neu5Gc in normal human and malignant tissues (Table 4).
Examination of frozen tissue sections showed expression in blood vessels, some epithelia, and in certain epithelial carcinomas [20, 110, 111]. Specificity of binding is confirmed by using a control IgY, and by inhibition using Neu5Gc rich chimpanzee serum. About 30% of breast, ovarian, and prostate carcinomas from humans showed expression of Neu5Gc (Fig. 8).
Other than expression within blood vessels within the malignant tissue, no expression was observed in melanomas and lymphomas using this three-step immunohistochemistry method. Paraffin sections showed a marked loss of expression, as did pre-incubation of frozen sections with methanol, indicating that the Neu5Gc epitopes are likely to be present mostly on glycolipids.
5 Conclusion and Perspectives
Structural diversity of Sia is not so large in each animal compared with that which we know in nature. In humans, Neu5Ac is a dominant Sia species, with NeuGc and Kdn contributing to only small amounts. 9-O-Acetylated Neu5Ac is a major modified Sia, but is still a minor form of total Neu5Ac in human. In rainbow trout, on the other hand, Neu5Ac, Neu5Gc, and Kdn appear to be equally expressed, although in a tissue-specific manner. Therefore, which Sia species each animal expresses for particular biological roles depends on the animal species. Evolutional pressures for the selection of Sia species may come from critical interactions for survival of life, such as pathogen–host interactions, and species-specific sperm–egg interactions in fertilization. Thus, in some cases, a particular Sia species is specifically functional; in other cases, no specific Sia is necessary. The presence of Kdn-specific sialidase in bacteria, the preferential recognition of Neu5Gc by mouse siglec-2, and an inhibitory effect of polySia, but not di- and oligoSia, on homophilic binding of NCAM may exemplify the significance of the structural diversity of Sia species. Importantly, modifications of Sia are often changed dynamically, including the O-acetylation/de-O-acetylation cycle and transient degradation of polySia to oligoSia. However, currently there is very little knowledge available about the enzymes involved in these modification/re-modification reactions. Future studies will be directed toward this identification of Sia-modification enzymes and their genes.
References
Angata T, Varki A (2002) Chemical diversity in the sialic acid and related α-keto acids: an evolutionary perspective. Chem Rev 102:439–469
Schauer R, Kamerling JP (2003) In: Montreuil J, Vliegenthart JFG, Schachter H (eds) Chemistry, biochemistry and biology of sialic acid, glycoproteins II. Elsevier, Amsterdam, pp 243–402
Varki A (1997) Sialic acids as ligands in recognition phenomena. FASEB J 11:248–255
Varki A, Hooshmand F, Diaz S, Varki NM, Hedrick SM (1991) Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell 65:65–74
Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, Kalloo G, Connole M, Shi HN, Varki N, Varki A, Pillai S (2009) B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med 206:125–138
Parameswaran R, Lim M, Arutyunyan A, Abdel-Azim H, Hurtz C, Lau K, Müschen M, Yu RK, von Itzstein M, Heisterkamp N, Groffen J (2013) O-Acetylated N-acetylneuraminic acid as a novel target for therapy in human pre-B acute lymphoblastic leukemia. J Exp Med 210:805–819
Mandal C, Schwartz-Albiez R, Vlasak R (2011) Functions and biosynthesis of O-acetylated sialic acids. Top Curr Chem. doi:10.1007/128_2011_310
Davies LRL, Varki A (2013) Why is N-glycolylneuraminic acid rare in the vertebrate brain? Top Curr Chem. doi:10.1007/128_2013_419
Hildebrandt H, Dityatev A (2013) Polysialic acid in brain development and synaptic plasticity. Top Curr Chem. doi:10.1007/128_2013_446
Wipfler D, Srinivasan GV, Sadick H, Kniep B, Arming S, Willhauck-Fleckenstein M, Vlasak R, Schauer R, Schwartz-Albiez R (2011) Differentially regulated expression of 9-O-acetyl GD3 (CD60b) and 7-O-acetyl-GD3 (CD60c) during differentiation and maturation of human T and B lymphocytes. Glycobiology 21:1161–1172
Lrhorfi LA, Srinivasan GV, Schauer R (2007) Properties and partial purification of sialate O-acetyltransferase from bovine submandibular glands. Biol Chem 388:297–306
Higa HH, Diaz S, Varki A (1987) Biochemical and genetic evidence for distinct membrane-bound and cytosolic sialic acid O-acetyl-esterases: serine-active-site enzymes. Biochem Biophys Res Commun 144:1099–1108
Shen Y, Kohla G, Lrhorfi AL, Sipos B, Kalthoff H, Gerwing GJ, Kamerling JP, Schauer R, Tiralongo J (2004) O-Acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem 271:281–290
Schauer R, Shukla AK (2008) Isolation and properties of two sialate-O-acetylesterases from horse liver with 4- and 9-O-acetyl specificities. Glycoconj J 25:625–632
Schwardt O, Kelm S, Ernst B (2013) SIGLEC4 antagonists. Top Curr Chem. doi:10.1007/128_2013_498
Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A (1998) A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA 95:11751–11756
Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A (1998) The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem 273:15866–15871
Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, Varki A (2010) Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med 207:1637–1646
Hedlund M, Padler-Karavani V, Varki NM, Varki A (2008) Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA 105:18936–18941
Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, Chen X, Witztum JL, Varki NM, Varki A (2009) Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood 114:5225–5235
Shilova N, Huflejt ME, Vuskovic M, Obukhova P, Navakouski M, Khasbiullina N, Pazynina G, Galanina O, Bazhenov A, Bovin N (2013) Natural antibodies against sialoglycans. Top Curr Chem. doi:10.1007/128_2013_469
Nadano D, Iwasaki M, Endo S, Kitajima K, Inoue S, Inoue Y (1986) A naturally occurring deaminated neuraminic acid, 3-deoxy-D-glycero-D-galacto-nonulosonic acid (KDN). Its unique occurrence at the nonreducing ends of oligosialyl chains in polysialoglycoprotein of rainbow trout eggs. J Biol Chem 261:11550–11557
Inoue S, Kitajima K (2006) KDN (deaminated neuraminic acid): dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj J 23:277–290
Inoue S, Kitajima K, Inoue Y (1996) Identification of 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid (KDN, deaminoneuraminic acid) residues in mammalian tissues and human lung carcinoma cells. Chemical evidence of the occurrence of KDN glycoconjugates in mammals. J Biol Chem 271:24341–24344
Go S, Sato C, Yin J, Kannagi R, Kitajima K (2007) Hypoxia-enhanced expression of free deaminoneuraminic acid in human cancer cells. Biochem Biophys Res Commun 357:537–542
Prokazova NV, Mikhailov AT, Kocharov SL, Malchenko LA, Zvezdina ND, Buznikov G, Bergelson LD (1981) Unusual gangliosides of eggs and embryos of the sea urchin Strongylocentrotus intermedius. Structure and density-dependence of surface localization. Eur J Biochem 115:671–677
Kochetkov NK, Smimova GP, Chekareva NV (1976) Isolation and structural studies of a sulfated sialosphingolipid from the sea urchin Echinocardium cordatum. Biochim Biophys Acta 424:274–283
Kubo H, Irie A, Inagaki F, Hoshi M (1990) Gangliosides from the eggs of the sea urchin, Anthocidaris crassispina. J Biochem (Tokyo) 108:185–192
Ijuin T, Kitajima K, Song Y, Kitazume S, Inoue S, Haslam SM, Morris HR, Dell A, Inoue Y (1996) Isolation and identification of novel sulfated and nonsulfated oligosialyl glycosphingolipids from sea urchin sperm. Glycoconj J 13:401–413
Kitazume S, Kitajima K, Inoue S, Haslam SM, Morris HR, Dell A, Lennarz WJ, Inoue Y (1996) The occurrence of novel 9-O-sulfated N-glycolylneuraminic acid-capped α2→5-O glycolyl -linked oligo/polyNeu5Gc chains in sea urchin egg cell surface glycoprotein. Identification of a new chain termination signal for polysialyltransferase. J Biol Chem 22:6694–6701
Miyata S, Sato C, Kitamura S, Toriyama M, Kitajima K (2004) A major flagellum sialoglycoprotein in sea urchin sperm contains a novel polysialic acid, an α2,9-linked poly-N-acetylneuraminic acid chain, capped by an 8-O-sulfated sialic acid residue. Glycobiology 14:827–840
Miyata S, Sato C, Kumita H, Toriyama M, Vacquier VD, Kitajima K (2006) Flagellasialin: a novel sulfated α2,9-linked polysialic acid glycoprotein of sea urchin sperm flagella. Glycobiology 16:1229–1241
Slomiany BL, Kojima K, Banas-Gruszka Z, Murty VL, Galicki NI, Slomiany A (1981) Characterization of the sulfated monosialosyltriglycosylceramide from bovine gastric mucosa. Eur J Biochem 119:647–650
Slomiany A, Kojima K, Banas-Gruszka Z, Slomiany BL (1981) Structure of a novel sulfated sialoglycosphingolipid from bovine gastric mucosa. Biochem Biophys Res Commun 100:778–784
Morimoto N, Nakano M, Kinoshita M, Kawabata A, Morita M, Oda Y, Kuroda R, Kakehi K (2001) Specific distribution of sialic acids in animal tissues as examined by LC-ESI-MS after derivatization with 1,2-diamino-4,5-methylenedioxybenzene. Anal Chem 73:5422–5428
Bulai T, Bratosin D, Pons A, Montreuil J, Zanetta JP (2003) Diversity of the human erythrocyte membrane sialic acids in relation with blood groups. FEBS Lett 534:185–189
Kawai Y, Takemoto M, Oda Y, Kakehi K, Ohta Y, Yamaguchi S, Miyake M (2000) Inhibition of in vitro fertilization of mouse gametes by sulfated sialic acid polymers. Biol Pharm Bull 23:936–940
Kimura K, Mori S, Tomita K, Ohno K, Takahashi K, Shigeta S, Terada M (2000) Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antiviral Res 47:41–51
Kisa F, Yamada K, Miyamoto T, Inagaki M, Higuchi R (2007) Determination of the absolute configuration of sialic acids in gangliosides from the sea cucumber Cucumaria echinata. Chem Pharm Bull(Tokyo) 55:1051–1052
Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC (1976) Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can J Biochem 54:1–8
Sato C, Kitajima K (2011) New functions of polysialic acid and its relationship to schizophrenia. Trends Glycosci Glycotechnol 23:221–238
Mühlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn R, Hildebrandt H (2013) Polysialic acid: versatile modification of NCAM, SynCAM 1 and Neuropilin-2. Neurochem Res doi:10.1007/s11064-013-0979-2
Sato C (2013) Polysialic acid. In: Tiralongo J, Martinez-Duncker I (eds) Sialobiology: structure, biosynthesis, and function, Bentham Science, pp 33–75. http://www.eurekaselect.com/107046/volume/1
Rutishauser U (2008) Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci 9:26–35
Kanato Y, Kitajima K, Sato C (2008) Complex formation of a brain-derived neurotrophic factor and glycosaminoglycans. Glycobiology 18:1044–1053
Kanato Y, Ono S, Kitajima K, Sato C (2009) Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Biotech Biosci Biochem 73:2735–2741
Sato C, Yamakawa N, Kitajima K (2010) Measurement of glycan-based interactions by frontal affinity chromatography and surface plasmon resonance. Methods Enzymol 478:219–232
Isomura R, Kitajima K, Sato C (2011) Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J Biol Chem 286:21535–21545
Ono S, Hane M, Kitajima K, Sato C (2012) Novel regulation of FGF2-mediated cell growth by polysialic acid. J Biol Chem 287:3710–3722
Vaithianathan T, Matthias K, Bahr B, Schachner M, Suppiramaniam V, Dityatev A, Steinhaüser C (2004) Neural cell adhesion molecule-associated polysialic acid potentiates α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J Biol Chem 279:47975–47984
Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A (2006) Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-D-aspartate receptors and prevents glutamate-induced cell death. J Biol Chem 281:34859–34869
Kambara Y, Shiba K, Yoshida M, Sato C, Kitajima K, Shingyoji C (2011) Mechanism regulating Ca2+-dependent mechanosensory behaviour in sea urchin spermatozoa. Cell Struct Funct 36:69–82
Sato C, Kitajima K (1999) Glycobiology of di- and oligosialyl glycotopes. Trends Glycosci Glycotechnol 11:371–390
Sato C (2004) Glycobiology of di- and oligosialyl glycotopes. Trends Glycosci Glycotechnol 16:331–344
Nadanaka S, Sato C, Kitajima K, Katagiri K, Irie S, Yamagata T (2001) Occurrence of oligosialic acids on integrin alpha-5 subunit and their involvement in cell adhesion to fibronectin. J Biol Chem 276:33657–33664
Sato C, Matsuda T, Kitajima K (2002) Neuronal differentiation-dependent expression of the disialic acid epitope on CD166 and its involvement in neurite formation in Neuro2A cells. J Biol Chem 277:45299–45305
Yasukawa Z, Sato C, Sano K, Ogawa H, Kitajima K (2006) Identification of disialic acid-containing glycoproteins in mouse serum: a novel modification of immunoglobulin light chains, vitronectin, and plasminogen. Glycobiology 16:651–665
Miyata S, Yamakawa N, Toriyama M, Sato C, Kitajima K (2011) Co-expression of two distinct polysialic acids, α2,8- and α2,9-linked polymers of N-acetylneuraminic acid, in distinct glycoproteins and glycolipids in sea urchin sperm. Glycobiology 21:1596–1605
Aminoff D (1961) Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J 81:384–392
Uchida Y, Tsukada Y, Sugimori T (1977) Distribution of neuraminidase in Arthrobacter and its purification by affinity chromatography. J Biochem (Tokyo) 82:1425–1433
Svennerholm L (1957) Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta 24:604–611
Hara S, Takemori Y, Yamaguchi M, Nakamura M, Ohkura Y (1987) Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem 164:138–145
Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M (1989) Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem 179:162–166
Yamakawa N, Sato C, Miyata S, Maehashi E, Toriyama M, Sato N, Furuhata K, Kitajima K (2007) Development of sensitive chemical and immunochemical methods for detecting sulfated sialic acids and their application to glycoconjugates from sea urchin sperm and eggs. Biochimie 89:1396–1408
Kamerling JP, Schauer R, Shukla AK, Stoll S, Van Halbeek H, Vliegenthart JF (1987) Migration of O-acetyl groups in N, O-acetylneuraminic acids. Eur J Biochem 162:601–607
Finne J, Krusius T, Rauvala H (1977) Occurrence of disialosyl groups in glycoproteins. Biochem Biophys Res Commun 74:405–410
Michon F, Brisson JR, Jennings HJ (1987) Conformational differences between linear α2,8-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 26:8399–8405
Sato C, Kitajima K, Tazawa I, Inoue Y, Inoue S, Troy FA II (1993) Structural diversity in the α2-8-linked polysialic acid chains in salmonid fish egg glycoproteins. Occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J Biol Chem 268:23675–23684
Sato C, Inoue S, Matsuda T, Kitajima K (1998) Development of a highly sensitive chemical method for detecting α2,8-linked oligo/polysialic acid residues in glycoproteins blotted on the membrane. Anal Biochem 261:191–197
Sato C, Inoue S, Matsuda T, Kitajima K (1999) Fluorescent-assisted detection of oligosialyl units in glycoconjugates. Anal Biochem 266:102–109
Zhang Y, Lee YC (1999) Acid-catalyzed lactonization of α2,8-linked oligo/polysialic acids studied by high performance anion-exchange chromatography. J Biol Chem 274:6183–6189
Nakata D, Troy FA II (2005) Degree of polymerization (DP) of polysialic acid (polySia) on neural cell adhesion molecules (N-CAMS): development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMS. J Biol Chem 275:38305–38316
Inoue S, Lin SL, Lee YC, Inoue Y (2001) An ultrasensitive chemical method for polysialic acid analysis. Glycobiology 11:759–767
Rohr TE, Troy FA (1980) Structure and biosynthesis of surface polymers containing polysialic acid in Escherichia coli. J Biol Chem 255:2332–2342
Kitajima K, Kuroyanagi H, Inoue S, Ye J, Troy FA 2nd, Inoue Y (1994) Discovery of a new type of sialidase, “KDNase,” which specifically hydrolyzes deaminoneuraminyl (3-deoxy-D-glycero-D-galacto-2-nonulosonic acid) but not N-acylneuraminyl linkages. J Biol Chem 269:21415–21419
Kanamori A, Inoue S, Xulei Z, Zuber C, Roth J, Kitajima K, Ye J, Troy FA 2nd, Inoue Y (1994) Monoclonal antibody specific for α2,8-linked oligo deaminated neuraminic acid (KDN) sequences in glycoproteins. Preparation and characterization of a monoclonal antibody and its application in immunohistochemistry. Histochemistry 101:333–340
Song Y, Kitajima K, Inoue Y (1993) Monoclonal antibody specific to α2,3-linked deaminated neuraminyl beta-galactosyl sequence. Glycobiology 3:31–36
Angata T, Matsuda T, Kitajima K (1998) Synthesis of neoglycoconjugates containing deaminated neuraminic acid (KDN) using rat liver α2,6-sialyltransferase. Glycobiology 8:277–284
Ohta K, Sato C, Matsuda T, Toriyama M, Lennarz WJ, Kitajima K (1999) Isolation and characterization of low density detergent-insoluble membrane (LD-DIM) fraction from sea urchin sperm. Biochem Biophys Res Commun 258:616–623
Sarff LD, McCracken G, Schiffer MS, Glode MP, Robbins JB, Ørskov I, Ørskov F (1975) Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet 1975:1099–1104
Frosch M, Gorge I, Boulnois GJ, Timmis KN, Bitter-Suremann D (1985) NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA 82:194–1198
Jennings HJ, Roy R, Michon F (1985) Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol 134:2651–2657
Häyrinen J, Bitter-Suermann D, Finne J (1989) Interaction of meningococcal group B monoclonal antibody and its Fab fragment with α2-8-linked sialic acid polymers: requirement of a long oligosaccharide segment for binding. Mol Immunol 26:523–529
Sato C, Kitajima K, Inoue S, Seki T, Troy FA II, Inoue Y (1995) Characterization of the antigenic specificity of four different anti-(α2,8-linked polysialic acid) antibodies using lipid-conjugated oligo/polysialic acids. J Biol Chem 270:8923–18928
Sato C, Fukuoka H, Ohta K, Matsuda T, Koshino R, Kobayashi K, Troy FA II, Kitajima K (2000) Frequent occurrence of pre-existing α2→8-linked disialic and oligosialic acids with chain lengths up to 7 Sia residues in mammalian brain glycoproteins. Prevalence revealed by highly sensitive chemical methods and anti-di-, oligo-, and poly-Sia antibodies specific for defined chain lengths. J Biol Chem 275:15422–15431
Inoko E, Nishiura Y, Tanaka H, Takahashi T, Furukawa K, Kitajima K, Sato C (2010) Developmental stage-dependent expression of an α2,8-trisialic acid unit on glycoproteins in mouse brain. Glycobiology 20:916–928
Yasukawa Z, Sato C, Kitajima K (2007) Identification of an inflammation-inducible serum protein recognized by anti-disialic acid antibodies as carbonic anhydrase II. J Biochem 141:429–441
Jakobsson E, Schwarzer D, Jokilammi A, Finne J (2012) Endosialidases – versatile tools for the study ofpolysialic acid. Top Curr Chem. doi:10.1007/128_2012_349
Hallenbeck PC, Vimer ER, Yu F, Basseler B, Troy FA (1987) Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-α-2,8-sialosyl carbohydrate units. J Biol Chem 262:3553–3561
Pelkonen S, Perkonen J, Finne J (1989) Common cleavage pattern of polysialic acid by bacteriophage endosialidases of different properties and origins. J Virol 63:4409–4416
Miyake K, Muraki T, Hattori K, Machida Y, Watanabe M, Kawase M, Yoshida Y, Iijima S (1997) Screening of bacteriophages producing endo-N-acetylneuraminidase. J Ferm Bioeng 84:90–93
Aalto J, Pelkonen S, Kalimo H, Finne J (2001) Mutant bacteriophage with non-catalytic endosialidase binds to both bacterial and eukaryotic polysialic acid and can be used as probe for its detection. Glycoconj J 18:751–758
Martin LT, Marth JD, Varki A, Varki NM (2002) Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem 277:32930–32982
Yamamoto K, Konami Y, Irimura T (1997) Sialic acid-binding motif of Maackia amurensis lectins. J Biochem 121:756–761
Kim BS, Oh TK, Cho DH, Kim YJ, Koo WM, Kong KH, Kom H (2004) A sialic-acid binding lectin from the legume Maackia fauriei: comparison with lectisn from M. amurensis. Plant Sci 167:1315–1321
Brinkmann-van der Linden ECM, Sonnenburg JL, Varki A (2002) Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis Hemagglutinin. Anal Biochem 303:98–104
Nicholls JM, Bourne AJ, Chen H, Guan Y, Malik Peiris JS (2007) Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 8:73–83
Imberty A, Gautier C, Lescar J, Perez S, Wyns L, Loris R (2000) An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J Biol Chem 275:17541–17548
Geisler C, Jarvis DL (2011) Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 21:988–993
Herrler G, Rott R, Klenk HD, Muller HP, Shukla AK, Schauer R (1985) The receptor-destroying enzyme of influenza C virus is neuraminate O-acetylesterase. EMBO J 4:1503–1506
Vlasak R, Krystal M, Nacht M, Palese P (1987) The influenza c virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology 160:419–425
Zimmer G, Reuter G, Schauer R (1992) Use of influenza C virus for detection of 9-O-acetylated sialic acids on immobilized glycoconjugates by esterase activity. Eur J Biochem 204:209–215
Klein A, Krishna M, Varki NM, Varki A (1994) 9-O-Acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc Natl Acad Sci USA 91:7782–7786
Shi WX, Chammas R, Varki NM, Powell L, Varki A (1996) Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem 271:31526–31532
Muchmore E, Varki A (1987) Selective inactivation of influenza C esterase: a probe for detecting 9-O-acetylated sialic acids. Science 236:1293–1295
Mehansho H, Carlson DM (1983) Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. J Biol Chem 258:6616–6620
Schwegmann-Webels C, Herrier G (2006) Sialic acids as receptor determinants for coronaviruses. Glycoconj J 23:51–58
Langereis MA, van Vliet ALW, Boot W, deGroot RJ (2010) Attachment of mouse hepatitis virus to O-acetylated sialic acid is mediated by hemaglutinin-esterase and not by the spike protein. J Virol 2010:8970–8974
Schauer R (1982) Sialic acids: chemistry, metabolism and function, cell biology monographs, vol 10. Springer, Berlin
Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E (2003) Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA 100:12045–12050
Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, Brinkman-Van der Linden EC, Varki A, Varki NM (2009) Sensitive and specific detection of the non-human sialic acid N-glycolyneuraminic acid in human tissues and biotherapeutic products. PLoS One 4:e4241
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kitajima, K., Varki, N., Sato, C. (2013). Advanced Technologies in Sialic Acid and Sialoglycoconjugate Analysis. In: Gerardy-Schahn, R., Delannoy, P., von Itzstein, M. (eds) SialoGlyco Chemistry and Biology II. Topics in Current Chemistry, vol 367. Springer, Cham. https://doi.org/10.1007/128_2013_458
Download citation
DOI: https://doi.org/10.1007/128_2013_458
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21316-3
Online ISBN: 978-3-319-21317-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)