Abstract
This opening chapter recalls the history of the discoveries that led to the appreciation of the nature and importance of molecular chirality in biology, as well as the development of stereochemistry as an interdisciplinary field connecting chemistry and biology. The discoveries described cover roughly the period of ca. 1840–1940, although certain relevant events of earlier or later times are also addressed. A large number of chiral substances occur in nature in unichiral (i.e., single-enantiomer) form, and for centuries many such substances were used in crude extracts for relief from diseases. For the science of biochirality, the first milestone was the discovery of molecular chirality by Louis Pasteur in 1848. Thereafter, fundamental advances were made, beginning in 1857 with Pasteur’s discovery of biological enantioselectivity, in the metabolism of (±)-tartaric acid. With the advances in organic chemistry during the second half of the nineteenth century, the structures of many organic molecules were elucidated and new chiral compounds synthesized, and by the turn of the twentieth century studies of stereoselectivity in the biological activity or enzymatic transformations of natural or synthetic substances were proliferating, and chiroselectivity was often found. Among the names associated with important discoveries in biochirality appear Pasteur, Piutti, Fischer, Cushny, Easson and Stedman, and others. The findings soon prompted attempts to explain the phenomenon of enantioselectivity in biological action, beginning with Pasteur’s proposal to account for enantioselectivity in the metabolism of tartaric acid. In 1894 Fischer announced his “lock-and-key” metaphor to explain enantioselectivity in enzyme-substrate interactions and in 1933 Easson and Stedman advanced the first chemical-structure-based model, the three-point-attachment paradigm, to rationalize enantioselectivity at adrenergic receptors. This model has been generalized as the simplest basis for enantioselectivity in biological activity. Today molecular chirality is widely recognized as an important modulator of the effects of chiral substances in a variety of branches of biology and medicine.
Similar content being viewed by others
Keywords
- Biological enantioselectivity
- Chirality
- Chiroselectivity
- Drugs
- History of chemistry pharmacology
- Molecular recognition
- Natural products
1 Introduction
Interest in the role of molecular chirality in biology is intense today [1–5]. It is abundantly clear by now that stereoisomeric chiral molecules often differ significantly – and at times drastically – in their biological effects and/or disposition. The most fascinating phenomenon in this domain is undoubtedly what is referred to as biological enantioselectivity, i.e., differences between enantiomerically related molecules in their biological effects or behavior. “Enantiomerically related molecules” refers, of course, to chiral molecules that are nonsuperposable-mirror-image forms of each other. Enantioselectivity has been observed in many areas of biology, e.g., biochemistry, physiology, pharmacology, toxicology, etc., and the phenomenon has important implications for these disciplines and the associated technologies. It is also true, however, that other types of stereoselectivity (i.e., collectively, diastereoselectivity) among chiral molecules in their biological effects exist and at times are important (for a brief discussion of the relevant terminology, see the following section in this chapter).
In this opening chapter the early history of the recognition of the role of molecular chirality in biology is examined. In this regard, some may ask “why should we pay attention to history”? Perhaps the most succinct answer to this question was given by the French writer and statesman André Malraux (1901–1976):
Qui veut lire
dans l'avenir
doit feuilleter
dans le passé.
(Whoever wants to read the future must peruse the pages of the past).
Although Malraux was not speaking in the science context, there is in fact little doubt that an examination of past scientific discoveries and the methods and thinking of scientists of the past can provide a perspective relevant and useful to current problems and future solutions. It is in this spirit, then, that we undertake in this chapter to “peruse the pages” of the rich history of molecular chirality in biology.
The chapter focuses on molecular aspects of biochirality, leaving aside issues related to left-right selection in embryogenesis and morphogenesis, even if these rely ultimately on molecular action. The emphasis will be on the interactions of small molecules with biological systems, and the history of the discoveries concerning the chirality per se of biological macromolecules (proteins, nucleic acids, etc.) will not be addressed here. Our approach in examining the history is to describe the important discoveries and their background and significance rather than provide an exhaustively comprehensive survey. After considering the millennia-old history of folk remedies based on chiral molecules, our analysis of the development of the science of molecular biochirality will cover the period of ca. 1840–1940, an era during which many of the fundamental observations were made. However, at times references will be made to earlier or more recent events relevant to the discussion.
2 Getting Started: A Semantic Touch
Stereochemistry, a complex and rich science, has a language that is therefore also rich and complex, and, naturally, a discussion of the stereochemical matters at hand requires the application of precise scientific language [6–11]. To state the obvious, accuracy in concepts requires accuracy in language. While a detailed discussion of the language of stereochemistry and its problems would be beyond the scope of this chapter, a few brief comments on some of the terminology essential to the topics included here would seem important.
Chiral was defined in one leading monograph on stereochemistry as follows: “Not superposable…with its mirror image, as applied to molecules, conformations, as well as macroscopic objects, such as crystals” [12]. Mislow gave a shorter but essentially equivalent definition: “An object is chiral if and only if it is not superposable on its mirror image; otherwise it is achiral” [13]. Thus, it is clear that chiral refers to a spatial property of objects, including molecules. Therefore, the term describes that nature of a molecule which makes it non-superposable on its mirror image, and it does not refer to the stereochemical composition of bulk material, i.e., drugs, compounds, substances, etc. Thus, “chiral compound” does not tell us whether the substance is racemic, a single enantiomer, or some other mixture of the stereoisomers. In the present chapter, therefore, chiral will be used strictly according to the definitions cited above, i.e., to refer to the chirality of individual molecules or other chiral objects. Thus, “chiral drug,” “chiral substance,” etc., will be used to indicate that the compound in question is composed of chiral molecules, but the enantiomer composition is not specified by this terminology. This point needs to be emphasized because the literature contains other, often troubling, uses of the term; for example, some have applied chiral to indicate that the substance in question consists of only one of the two enantiomers, a usage that often leads to confusion. Similarly, the expression chiral non-racemic is entirely vague since it might allude either to a sample that consists of only one of the enantiomers or to one containing both enantiomers in undefined ratio but not racemic.
There is, however, a genuine and obvious need for a convenient term to refer to chiral substances that are composed of only one of the two enantiomers. Numerous terms for this purpose have been introduced over many years, but a consensus on this matter has not been reached. One of the present authors has discussed this issue in detail and introduced a new term for the purpose: unichiral [14]. In the present chapter unichiral will be used to specify the stereochemical composition of a chiral substance, compound, sample, etc., as stereochemically homogeneous, i.e., a single substance consisting of the same chiral molecules (single enantioform), in the context of use and within the limits of measurement [14]. “In the context of use” is an important element here, since “single-stereoisomer” identity is a variable concept, defined by the nature of the particular context in which it is used. To illustrate, a sample of levodeoxyephedrine [(R)-N,α-dimethylbenzeneethanamine], a “unichiral” over-the-counter medication used for the relief of nasal congestion, has been found to contain 1% contamination with the dextrorotatory enantiomer [15]. In the context of the use of this product, however, this level of enantiopurity is acceptable (the presence of 1% of the dextro enantioform has no pharmacological or clinical significance) and the drug is indeed considered “unichiral.” On the other hand, when testing for the beta-adrenergic-antagonist activity of (+)-(R)-propranolol (a very weak antagonist), a 1% contamination with the levo enantiomer (which is 100 times more potent than (+)-propranolol as an antagonist) would distort the results of the testing and would thus be unacceptable. That is, the sample of (+)-propranolol containing 1% of the levo enantiomer could not be called “unichiral” in the context considered.
An important aspect of many chiral molecules from nature is their homochirality. This term, coined by Lord Kelvin in 1894 [16], indicates the same sense of chirality among chiral objects or molecules, i.e., their chirality is of the same direction or configuration. Thus, D-alanine and D-serine are homochiral, i.e., they have the same sense of chirality (configuration). Homochirality is a commonly seen phenomenon among chiral molecules from nature. This means that related chiral natural molecules often have the same configuration. For example, with relatively few exceptions α-amino acids occurring in nature consistently have the L configuration; similarly, most naturally occurring monosaccharides are of the D configuration, etc. Thus, both unichirality and homochirality are typical for compounds from nature: most of them occur in single-enantiomer form, and closely related molecules usually have the same sense of chirality. Unfortunately, the usage of homochiral (and homochirality) in the literature is fraught with inconsistencies and contradictions [14]. Above all, the most pernicious misuse of homochiral is as a synonym of enantiomerically pure. Be that as it may, in the present chapter homochiral will be used as defined above, i.e., indicating the same sense of chirality when comparing similar or related molecules.
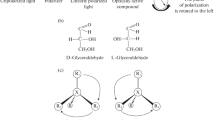
Another term that needs to be addressed in the present context is molecular asymmetry. This expression is often employed to refer to molecular chirality, but such usage, in which “asymmetry” is equated with “chirality,” is incorrect. To put it succinctly: an asymmetric object is necessarily chiral, but a chiral object is not necessarily asymmetric. An object or molecule is asymmetric if it has no symmetry element other than the identity operation (E or I), i.e., belonging to the (trivial) point group C1. Such a structure is necessarily incongruent with (i.e., not superposable on) its mirror image, that is, chiral. However, the presence in a structure of some symmetry, namely, one or more simple axes of symmetry (i.e., proper rotation axis, Cn, n > 1), does not preclude chirality. For example, the enantiomers of trans-1,2-dimethylcyclohexane are chiral but not asymmetric since they contain a C2 axis of symmetry. This means that 180° rotation of the molecule around the axis results in the same molecule, superposed on the original (Fig. 1).
Thus an object or molecule may be chiral without being asymmetric, and therefore “asymmetry” and “chirality” are not synonyms and should not be conflated [9]. Overall, “molecular asymmetry,” when it is employed to refer specifically to the phenomenon of molecular chirality, should in fact be replaced with “molecular chirality.”
Other relevant stereochemical vocabulary includes stereoselective, enantioselective, enantiospecific, and related terms. “Stereoselective” refers to selectivity concerning stereoisomers, i.e., a general term that does not identify the type of stereoisomers involved. “Enantioselective” and “enantiospecific” obviously refer to discrimination of or by enantiomerically related substances, but the distinction between the two terms is not clear. In this chapter “enantioselective” will be used.
Diastereoisomers are stereoisomers that are not related as object and mirror image, and may be chiral or achiral. Several significant examples of biological diastereoselectivity between chiral diastereoisomers will be discussed in this chapter. The terms “chiroselective” and “chiroselectivity” have appeared in the literature [17, 18] but are infrequently used. They refer to discrimination between chiral stereoisomers, be they enantiomers or diastereoisomers. Thus, they are useful terms when discrimination specifically between chiral stereoisomers is the intended meaning, without however limiting the phenomenon to enantioselectivity or diastereoselectivity.
3 Chiral Natural Products as Folk Remedies: The “Pre-science” Era
For thousands of years, substances obtained from vegetable, animal, or mineral sources were relied upon for relief from human diseases [19]. Such folk medicine was by its very nature inaccurate and unscientific and often had no rational basis. Moreover, toxicity of many of the products was a serious problem; indeed, some of the pharmacologically active preparations were used as poisons. The advent of the printing press in the fifteenth century resulted in the wide dissemination of knowledge about natural medications and this in turn produced a considerable increase in the use, and misuse, of such remedies. More rational therapy with purified natural products began only in the 1800s [19].
Despite the problems, however, some of the natural preparations were effective in relieving the symptoms and at times even eliminating the disease. In fact, we know today that the number of pharmacologically active substances produced by nature is large and the spectrum of biological activities of natural products is extraordinarily broad; for example, antimicrobial, antineoplastic, CNS-active, anti-inflammatory, cardiovascular, etc., are only a few of the therapeutic classes of drugs from nature.
Chirality is a hallmark of many molecules from nature. Indeed, the number of chiral natural molecules is very large and the structural variety they represent is vast. Among such substances – be they small molecules or macromolecules – an overwhelming majority occur in unichiral form. For example, in general, chiral α-amino acids and the peptides and proteins containing them, sugars and their polysaccharides, steroids, antibiotics, and many other compounds from nature are unichiral. It should be emphasized, however, that unichirality in natural products is not an absolute rule, and occasionally both enantiomers may be formed in the same or different genera/species. In particular, secondary metabolites may occur in both enantiomeric forms and are sometimes isolated as either the racemic or a scalemic mixture (i.e., enriched in one of the enantiomers) [20].
In light of the above, it is not surprising that many of the compounds used as therapeutic agents in natural remedies over the centuries and millennia have been chiral and that the vast majority of such substances occur in unichiral form. For centuries, until the beginning of the nineteenth century, most such natural remedies were used as crude plant extracts rather than purified active principles. Obviously, in that “pre-scientific” era, the remedies were used without any knowledge of the nature or identity of the active ingredient(s) within, let alone any understanding of the chirality of the molecules involved. Recognition of the existence of chirality in natural products had to await a better understanding of chemical structure, i.e., the advent of modern structural organic chemistry, and the discovery of molecular chirality (see below).
The number of pharmacologically active agents now known to be present in various old remedies is large [21] and many of these compounds are based on chiral molecules. Information about some of the earliest herbal remedies that today are known to contain chiral active ingredients goes back nearly 5,000 years. A few examples of old therapies with chiral active ingredients are presented below.
In a book about herbs, the Chinese scholar-emperor Shen Nung described in 2735 B.C. the beneficial effects of Ch'ang Shan in the treatment of “fevers” [22]. This preparation is the powdered root of a plant, Dichroa febrifuga Lour. Modern medicinal chemistry has identified several alkaloids with antimalarial properties in the plant, and it is therefore clear that the ancient use of Ch'ang Shan in fevers was not entirely without basis. One of the antimalarial compounds from Ch'ang Shan is februgine (β-dichroine, 3-[3-[(2R,3S)-3-hydroxy-2-piperidinyl]-2-oxopropyl]- 4(3H)-quinazolinone), a relatively simple unichiral compound. Modern attempts to develop these agents as antimalarial drugs failed due to significant toxicity [22].
Shen Nung also observed the stimulant properties of another Chinese plant, Ma Huang, now known as Ephedra sinica [23]. The chief active ingredient, ephedrine ((αR)-α-[(1S)-1-(methylamino)ethyl]benzenemethanol) is a unichiral levorotatory sympathomimetic amine, and therefore it is also clear in this case that the use of Ma Huang as a stimulant had a rational basis. Ephedrine was first isolated from Ma Huang in 1887 [24], i.e., more than 4,600 years after the effects of the compound were recorded. Ephedrine was introduced into medical practice during the 1920s [25] and for decades was widely used – as a CNS stimulant in narcolepsy, as a bronchodilator, in the treatment of Adams-Stokes syndrome with complete heart block, as a stimulant in some forms of depression, and in some other disorders – but more recently it has been largely replaced in most of these indications by other treatment modalities [26]. Ephedrine has also been widely available in “dietary supplements” for weight loss, increased energy, body building, etc. However, in the early 1990s concern arose over potentially serious adverse effects from such use of ephedrine, including cardiovascular, nervous-system, and other toxic effects, and in April 2004 the U.S. Food and Drug Administration (FDA) banned the sale in the United States of dietary supplements containing ephedrine or closely related compounds [27].
Another millennia-old unichiral drug is the opioid agent morphine. Opioid refers broadly to all compounds related to opium (a more recent definition states that the term opioid includes any compound that interacts with the brain’s opioid receptors [28]). Opium powder is the dried juice from the unripe seed capsule of the poppy Papaver somniferum and its name is derived from the diminutive of the Greek word όπός (opos), i.e., vegetable juice. Opium has analgesic, euphoric, and other effects and contains many alkaloids, including morphine and codeine. Poppy juice is mentioned in the writings of the Greek philosopher and naturalist Theophrastus (ca. 371–287 B.C.), but evidence has been found suggesting that opium may have been known much earlier to ancient civilizations in Egypt and Mesopotamia [28, 29].
Within the Arab-Islamic civilization, whose rise began in the seventh century, opium came to be used mainly as a constipant to control dysentery [30]. The arrival of the Islamic armies and influence in Europe in the sixteenth century (Constantinople fell to the Ottoman Turks in 1453 and the first siege of Vienna by the Ottoman army took place in 1529) brought opium to Europe. Laudanum, a somewhat purified opium concentrate, was compounded by Paracelsus (Theophrastus Bombastus von Hohenheim, 1493–1541), a Swiss alchemist and physician, and the smoking of opium became openly popular during the 1700s; however, opium may have been extensively but less openly used in Europe in earlier times [31].
The unichiral natural substance (−)-morphine, the most important alkaloid in opium, was obtained as a purified powder from opium in 1805 by Sertürner and he described his findings in detail in 1817 [32]. He named it morphium after Morpheus, the Roman god of dreams, so named by Ovid using a Greek word. Later, the eminent French chemist and physicist Joseph-Louis Gay-Lussac (1778–1850), who was a strong supporter of Sertürner in his priority claim for the isolation of the substance over French pretenders, renamed the drug morphine. The chemical structure of morphine (without the absolute configuration) was elucidated in 1923 [33].
The invention of the hypodermic needle and syringe in the middle of the nineteenth century resulted in the widespread use of morphine, and addiction became a common problem. An early – and false – hope to circumvent the addiction liability of morphine was provided by a most unlikely candidate, heroin. This compound, the diacetyl derivative of morphine, is a potent opiate narcotic first synthesized in 1874 via acetylation of morphine, and was introduced into medical practice in 1898 as a cough suppressant [24]. Heroin is a semisynthetic drug, i.e., a chemically modified derivative of a natural product, and retains the stereochemistry of morphine. Heroin may have been the first synthetic unichiral drug introduced in clinical medicine. Heroin was actively marketed to physicians by its manufacturer (Bayer).
Heroin was touted as a “non-addicting” morphine analog that could safely replace morphine and thereby eliminate the latter’s addiction problem [34]. This claim turned out to be tragically mistaken and the abuse of heroin rapidly became widespread. A report in the Journal of the American Medical Association in 1912 warned of heroin abuse and called for strict legislation to prevent its sale in drugstores at a price of less than $1 per one hundred tablets (30 mg each) [35]. As is well known, such warnings had little effect, and today heroin is the most important abused opioid, with grave social, economic, and medical consequences.
Another chiral drug, methadone, a totally synthetic opiate agonist, has been recruited to fight heroin addiction. Methadone was first synthesized, in the racemic form, in Germany (at the IG Farbenindustrie company) just before WWII and was later shown to have stereoselective opioid agonist properties, concentrated nearly exclusively in the levorotatory enantiomer [36]. Methadone is widely used in the racemic form as an analgesic and in the treatment of opiate addiction, but in some other countries the pharmaceutical product is the unichiral levo form [37].
Perhaps the most fascinating old chiral drug, from a historical point of view, is the antimalarial agent quinine. Its earliest history is obscure, but it is known that by the early 1600s it was being used by South American natives in Peru, Ecuador, and neighboring regions as a crude preparation from the bark of a local tree for the treatment of “fevers.” In 1633 Antonio de la Calancha (1584–1654), an Augustinian monk in Lima, Peru, wrote: “There is a tree of ‘fevers’ in the land of Loja, with cinnamon-colored bark of which the Lojans cast powders which are drunk in the weight of two small coins, and [thereby] cure fevers and tertians; [these powders] have had miraculous effects in Lima” [38] (in some texts Loja is spelled Loxa). This appears to be the first recorded mention of the powers of the tree (later named Cinchona by the Swedish botanist Linnaeus) used by the natives to cure “fevers.” By the middle of the 1600s the extract of “Jesuit’s bark” (one of the names cinchona came to be known by) was being used in Europe indiscriminately for a variety of fevers. Cinchona was, however, effective only against malaria, an infectious disease widespread in many regions of Africa and Asia, and even in Europe and North America for centuries. Cinchona was the first effective treatment for malaria, and in 1820 the French pharmacists Pierre Joseph Pelletier (1788–1842) and Joseph Bienaimé Caventou (1795–1877) isolated quinine, the main antimalarial ingredient, from cinchona bark [39]. After its isolation in 1820, purified quinine quickly replaced the crude cinchona preparations in the treatment of malaria.
As mentioned above, the name cinchona was coined by the Swedish botanist Linnaeus (Carl von Linné, 1707–1778) in honor of Doña Francisca Henriquez de Ribera, the fourth Condesa (Countess) of Chinchón and wife of the viceroy of Peru, a Spanish colony at the time [40]. According to legend, in 1638 she was cured of malaria by the bark and, impressed with the cure, she took samples of cinchona to Spain, thereby launching the European career of the miracle remedy. However, as has been frequently pointed out, there are problems with Linnaeus’ nomenclature. First, he misspelled cinchona, leaving out the first h present in the countess’ name; second, she could not have taken the cinchona bark to Europe since she died in South America, in Cartagena de Indias (located today in Colombia) before she could return to Spain [40]. Be that as it may, cinchona has stuck in the official names of several species (e.g., Cinchona officinalis L and other species in the Rubiaceae family). As for quinine, this name is derived from quina quina (“bark of barks”), the Spanish spelling of a native Quechua name that was sometimes used for the cinchona tree in Peru, and was given by Pelletier and Caventou to their new substance [41].
After purified quinine became available during the first half of the nineteenth century, demand for the drug became intense, since malaria was widespread and quinine was needed in Europe, North America, and in various parts of Africa, Asia, and the Americas, where the major powers were engaged in establishing or strengthening their colonial control (indeed, quinine is tarnished with the infelicitous legacy of having facilitated colonization in some parts of the world). However, the supply of quinine was limited, and therefore the chemical synthesis of the substance became a topic of considerable interest to chemists. In England in 1856 an 18-year old chemistry student named William Henry Perkin (1838–1907), working with August Wilhelm von Hofmann (1818–1892), a German professor of chemistry and director of the Royal College of Chemistry in London, attempted to synthesize quinine by oxidizing N-allyltoluidine with potassium dichromate. The reaction, predictably in hindsight, did not produce quinine, but Perkin’s further studies of the reaction led to the discovery of mauveine, a purple dye which in turn launched the “aniline” or “coal-tar” dyes and the synthetic-dye industry. Moreover, the invention of mauveine not only revolutionized the dye and textile industries but also produced an intense stimulatory effect on chemical research in general, on the pharmaceutical industry, and on medicine [42] (Perkin’s mauveine is a mixture of several achiral compounds).
Malaria was eventually eradicated in North America by the late 1940s and in Europe by the mid-1970s, as a result of better insect control, better construction and insulation of homes and buildings (to exclude mosquitoes), and the draining of swamps, marshes, and other bodies of stagnant water (where mosquitoes reproduce). However, malaria remains a rampant affliction in many other parts of the world, with nearly one million victims dying of the disease every year, the majority of them African children under the age of five [43].
The chemical structure of quinine (without the stereochemical details) was established at the beginning of the twentieth century [44]. The first (formal) synthesis of quinine was achieved by Woodward and Doering during World War II [45]. Their synthesis, although of no commercial value, nevertheless attracted a great deal of attention at the time, since during the war the Allies were suffering from a severe shortage of quinine, needed for the protection and treatment of troops fighting in malarious regions. The shortage was the result of the fact that large-scale cultivation of cinchona had by then shifted primarily to Java (now part of Indonesia), a Dutch colony occupied by Japan during the war. The first fully stereoselective synthesis of quinine appeared in 2001 [46]. However, for commercial purposes, quinine continues to be obtained from its natural source, since the chemical syntheses are complex and therefore unsuitable for the large-scale production of the substance. The chiroselectivity aspects of the biological properties of quinine and related substances will be discussed in a later section of this chapter.
Another chiral drug from the New World worthy of mention here is curare, whose known history spans several centuries. The first reference to the substance was made early in the 1500s by Pietro Martire d’Anghiera, a chronicler to King Charles V of Spain who described a soldier being lethally wounded by an arrow tipped with poison. For several centuries thereafter, travelers to South America wrote about the poison. For example, Laurence Keynes, serving with Sir Walter Raleigh on his expedition to the current region of Venezuela, listed numerous poisons used by the natives, including a herb known as “ourari” (from which the name curare emerged) which caused paralysis [47, 48]. French physician and physiologist Claude Bernard reported that curare prevented the ligated limb of a frog from responding to stimulation, even though both the nerve and muscle retained their function [49]. In 1936 it was shown that acetylcholine acts as neurotransmitter at the neuromuscular junction and that curare is an antagonist of that action [50]. Clinical applications of curare during the nineteenth and first-half of the twentieth centuries in the treatment of convulsive diseases were largely hindered by the poor quality of the curare extracts. The use of curare in combination with anesthetics emerged only in the early 1940s and permitted the use of low doses of the latter while attaining an appropriate muscle relaxation [51]. The bioactive substance of curare is a dextrorotatory macrocycle of the bis(benzylisoquinoline) alkaloid family. Chemists at the Squibb company isolated this substance in 1943 from a sample from the New York Botanical Gardens [52], and the compound proved to be identical to a substance obtained earlier from a curare specimen stored in the British Museum [53]. Since the latter sample was packed in a bamboo tube, the drug was named tubocurarine.
The devastating disease scurvy is caused by insufficient amounts of L-ascorbic acid (vitamin C) in the diet. After the fifteenth century, exploration, expanding trade, and colonization by European powers required long sea voyages, usually undertaken without foods rich in vitamin C on board. The result was the decimation of ships’ crews by scurvy. In a remarkable study in 1747 that can be described as the first serious clinical therapeutic trial, British physician James Lind (1716–1794), a surgeon in the Royal Navy and the “father of naval hygiene,” demonstrated that fruits such as oranges and lemons can reverse and prevent the disease.
However, it was nearly 50 years later, in 1795, that the British Admiralty finally took notice of these findings and instituted an appropriate diet on board Royal Navy ships to prevent scurvy [54]. Ascorbic acid was isolated by the Hungarian biochemist Albert Szent-Györgyi (1893–1986) from fruit juices in 1928 and in part for this work he was awarded the Nobel Prize in Physiology or Medicine in 1937. In that same year one half of the Nobel Prize in Chemistry went to the English chemist Walter Norman Haworth (1883–1950) for the proof of structure and synthesis of ascorbic acid.
The above examples of old chiral drugs from natural sources are but a handful from a long list of many examples. Others include (some plant origins given in parentheses) tetrahydrocannabinol (marihuana, hashish), digoxin (foxglove, Digitalis lanata Ehrh.), cocaine (erythroxylon), cathinone (khat, Catha edulis Forsk.), nicotine (tobacco, Nicotiana tabacum), atropine (deadly nightshade, Atropa belladonna L.), reserpine (Rauwolfia), colchicine (autumn crocus, meadow saffron), and emetine (ipecac), to name only a few. Each of these chiral compounds has an interesting history but these accounts are beyond our scope here. The chemical structures encompassed by just these relatively few chiral molecules are highly varied. Stereochemically, atropine is an interesting case: this racemic substance is believed not to occur naturally, but its levorotatory form, (S)-(−)-hyoscyamine, occurs in several Solanaceae plant species and is racemized to atropine during isolation [55, 56].
The vast majority of chiral drugs present in the old remedies were unichiral substances: Mother Nature is not even-handed. All in all, chiral drugs have been of great importance in the development of pharmacotherapy, from the earliest plant remedies of millennia ago to the modern age. Many of these ancient chiral drugs are still in use today, and many new and important drugs have been developed by modifying the molecules of natural products identified in old remedies. The “pre-science” era of crude natural remedies came to an end as the nineteenth century was winding down. The birth of the modern era of therapeutics did not mean, however, the end of the therapeutic use of natural compounds, only that the science and technology became different.
At this stage, the development of the science of molecular biochirality required the recognition of the existence of the fundamental phenomenon of molecular chirality. This key discovery was made in the middle of the nineteenth century in France.
4 Discovery of Molecular Chirality
The relevant background work that led to the recognition of the existence of molecular chirality was accomplished, mainly in France, during the first half of the nineteenth century [57]. Hemihedrism in crystals – those of quartz – was first reported by René-Just Haüy (1743–1822), a French priest and crystallographer, in 1801 [58]. Circularly polarized light (often referred to as plane-polarized light) was discovered in 1809 by Étienne Louis Malus (1775–1812), and the physicist François Arago (1786–1853) made the first observation of optical rotation by a substance when he studied the effects of quartz crystals on polarized light [57].
French physicist Jean-Baptiste Biot (1774–1862) discovered in 1815 that certain natural organic compounds rotate polarized light in the non-crystalline state, e.g., in the liquid or solution state. Among these compounds were sucrose, turpentine, camphor, and tartaric acid (TA) [57]. TA – obtained from tartar deposits produced by the fermenting juice of grapes during the wine-making process – had been discovered by the Swedish pharmacist Carl Wilhelm Scheele (1742–1786) in 1769 [59], and Biot showed that the compound was optically active [60]. Biot understood that optical rotation by substances in the non-crystalline state was the result of some molecular property, but the recognition of molecular chirality was arrived at later by Biot’s younger colleague and protégé, Louis Pasteur (1822–1895) (Fig. 2).
Pasteur’s official photograph as member of the Académie française. Reproduced from http://academie-francaise.fr/immortels/index.html, courtesy of the Académie française
Pasteur earned a doctorate in physical sciences in 1847 at the faculty of sciences of the University of Paris. For the doctorate he submitted two dissertations, one in chemistry and one in physics. After earning the doctorate, he continued research work in the laboratory of Antoine-Jérôme Balard (1802–1876), an eminent chemist of the time. Pasteur focused his attention on questions of crystallography and optical rotation. He was familiar with the work by Biot outlined above on optical rotation by natural organic compounds, and in 1848 he found that the crystals of sodium ammonium tartrate (from the natural dextro-TA, Fig. 3) were hemihedral, i.e., there were small facets at alternate corners of the crystals [61], and he recognized that these facets rendered the crystals chiral.
Pasteur then examined the sodium ammonium salt of another, related, acid. That acid had been obtained ca. 1819 – unexpectedly and on a single occasion – as a side-product during the manufacture of (+)-TA from tartar at a chemical plant in Thann in Alsace, France [62]. The mysterious new acid intrigued chemists. Gay-Lussac obtained a sample for study and showed it to have the same composition as “ordinary” (dextrorotatory) TA; he named it racemic acid, from the Latin racemus, i.e., cluster of grapes [63]. Another name used for the compound was paratartaric acid. In most of its properties racemic acid was found to be identical with (+)-TA, with the exception of its crystal morphology and that it did not rotate polarized light, a fact first shown by Biot [64].
Pasteur obtained a sample of paratartaric/racemic acid from Charles Kestner, owner of the Thann plant [62, 65], and found – to his initial dismay – that the crystals of sodium ammonium racemate, like those of the corresponding “ordinary” (dextrorotatory) tartrate, were hemihedral (he had predicted that the crystals of the optically inactive acid would not be hemihedral or chiral). To his surprise, however, he observed that there were two different crystals present in the salt of racemic acid. That is, in some of the crystals the hemihedral facets were inclined to the right while in the others to the left, and Pasteur recognized that the two tartrate crystals were related to each other as the two hands, i.e., they were enantiomorphous (by today’s terminology). Pasteur then manually separated the two kinds of crystals and found that they rotated polarized light in solution, the rotations by the two being equal in absolute value (within experimental error) but opposite in direction. The dextrorotatory salt thus obtained was identical in all respects to the corresponding salt of the known (+)-TA and could be converted to a free acid that was identical in all respects to (+)-TA, while the levorotatory salt gave an acid that was identical to the natural acid except that it rotated polarized light in the opposite direction and its crystals were enantiomorphous with the natural acid. These results led Pasteur to realize that the molecules of the two substances in racemic acid must be chiral, due to some three-dimensional feature of their molecular structure, and that they are non-superposable-mirror-image (i.e., enantiomeric) molecules [61]. Pasteur, aged 25, announced his discovery in a lecture to the Académie des sciences in Paris on May 22nd, 1848 [61, 66]. Later he even proposed that a tetrahedral or helical arrangement of the atoms in the chiral molecules may be the basis of their chirality [67], proposals we now know to be correct.
Concerning Pasteur’s term for handedness, he did not use chirality – this term was coined, by Lord Kelvin in 1894, nearly 50 years after Pasteur’s discovery [16]. Pasteur did recognize the need for a specific term for handedness in molecules and objects and adapted the little-used French term dissymétrie (dissymmetry) to the phenomenon that today we call chirality [8].
Pasteur’s discovery also opened the road toward an appreciation of the widespread existence of molecular chirality in natural products. The first steps on that road were in fact taken by Pasteur himself. In the early 1850s he went on to study many chiral natural compounds, and he recognized that these molecules were chiral and that the substances isolated from their natural sources were unichiral [68]. Later, Pasteur stated the essence of the matter: “…morphine, codéine, quinine, strychnine, brucine,…Tous ces principes immédiats sont moléculairement dissymétriques” (…morphine, codeine, quinine, strychnine, brucine,… All these natural compounds have molecular dissymmetry) [67].
It should be mentioned here that in 1820, i.e., well before Pasteur, there was a theoretical suggestion that some molecules may lack symmetry [69]. It came from Sir John Frederick William Herschel (1792–1871) [70, 71], an eminent English astronomer, physicist, and chemist. He proposed that such lack of symmetry may be the explanation for Biot’s observation (see above) that some substances are optically active in the non-crystalline state. In 1827 Herschel repeated his suggestion, stating that such molecules “must be conceived as unsymmetrically constituted, i.e., as having a right and left side” [72]. This is, no doubt, a seed of the concept of molecular chirality. It must be recognized, however, that, insightful as it was, Herschel’s proposal of “unsymmetrical molecules” was limited in scope and remained only a theoretical suggestion: he did not elaborate further on the subject and did not pursue any experimental studies on the phenomenon. Moreover, Herschel did not dispute Pasteur’s claims for the discovery of molecular chirality. It was indeed Pasteur (whether he had been aware of Herschel’s ideas or not) who placed the concept of molecular chirality on a solid experimental foundation in a series of studies that required a great deal of him: the recognition of the fundamental problem of the sodium ammonium tartrate/paratartrate crystals, the design and execution of crucial and difficult experiments, exceptional powers of observation, thorough familiarity with the literature, and superior scientific intuition. Ultimately, based on his tartrate work, Pasteur was able to elaborate a fundamental chemical phenomenon that his eminent predecessors, e.g., Biot, Mitscherlich, de La Provostaye, Hankel, etc., all of whom had worked with tartrate crystals and had access to Herschel’s publications, had failed to recognize. All in all, it is therefore not inaccurate to speak of “Pasteur’s discovery of molecular chirality.” (Herschel’s proposal was recently discussed in detail [8]).
Pasteur’s preparation of levo-TA in 1848 produced the first known example of the existence of both enantiomers of a chiral substance. Shortly thereafter, in 1851, the isolation of (−)-camphor from a natural source by Chautard [73] created the second example [(+)-camphor had been known for a long time].
The next fundamental development in the history of molecular chirality occurred in 1874, when the asymmetric carbon atom was proposed as a basis for molecular chirality by the Dutch and French chemists Jacobus Henricus van't Hoff (1852–1911) [74] and Joseph Achille Lebel (1847–1930) [75], respectively, independently and almost simultaneously. They also proposed that the four substituents connected to the carbon atom were arranged in the shape of a tetrahedron. The discovery of the “asymmetric carbon atom” (van't Hoff’s terminology) finally provided the explanation for the existence of “optical isomers” and for the chiral nature of the molecules of optically active substances, including many naturally occurring substances.
5 Recognition of Biological Enantioselectivity
When in 1848 Pasteur began his experiments that led to the discovery of molecular chirality, all known optically active compounds were from natural sources. He recognized this fact and believed that optical activity is intimately connected to the genesis of such substances in nature, and ca. 10 years later he made a fundamental discovery relating the role of molecular chirality to biology.
Although he began his career as a chemist, Pasteur is primarily remembered today as the scientist who made revolutionary discoveries in microbiology and infectious diseases that have been of immense benefit to humanity. The circumstances and apparent reasons for his change of research direction from chemistry and crystallography to microbiology in the mid-to-late 1850s have recently been critically discussed and do not need to be further elaborated here [76]. Suffice it to state that in 1854, Pasteur, who was then professor of chemistry at Strasbourg, accepted an appointment as professor of chemistry and dean of the newly opened Faculty of Sciences at the University of Lille, in northern France. This was an industrial region where agricultural and food industries had considerable economic significance, and fermentation-based manufacturing, such as the production of ethanol from sugar beets and the production of beer, were of particular importance. It is highly likely that his move to Lille played a significant role in his shift to microbiology, including the study of fermentations [76].
After 3 years in Lille, Pasteur moved again, this time to Paris. On October 22nd, 1857, he was appointed Administrator of the École normale supérieure (ENS) and Director of Scientific Studies there. On December 21st, 1857, shortly after his arrival in Paris, he presented a communication to the Académie entitled “Memoir on Alcoholic Fermentation” which was published as a memoir in the proceedings of the Académie, the Comptes rendus des séances de l'Académie des Sciences (Comptes rendus henceforth) [77]. As its title indicates, the memoir dealt with certain aspects of alcoholic fermentation, but near the end of the communication Pasteur said the following:
Before concluding, I ask for the permission of the Academy to present results to which I attach great importance. I have discovered a means of fermenting tartaric acid which readily affects ordinary right tartaric acid but involves left tartaric acid very poorly or not at all. Now, a remarkable thing, predictable from the preceding fact, is that when paratartaric acid, formed by the combination, molecule for molecule, of the two tartaric acids, right and left, is subjected to the same method of fermentation, it is resolved into the right acid which is fermented and left acid which remains intact, in such a way that the best means of obtaining left tartaric acid I know of today is to resolve paratartaric acid by fermentation.
The description of the fermentation of “paratartaric acid” in the memoir of December, 1857, constitutes the first published observation of enantioselectivity in a biological process [77]. Approximately 3 months after that brief announcement of the enantioselective microbial metabolism of TA, Pasteur presented to the Académie a communication devoted entirely to the subject. The new communication, bearing the title “Memoir on the Fermentation of Tartaric Acid,” was presented to the Académie on March 29th, 1858, and, as usual, was published in the Comptes rendus [78]. Memoirs of original research appearing in the Comptes rendus were often relatively short, with few experimental details, and concentrated mainly on the essence and interpretation of the work. Pasteur sometimes followed up a presentation to the Académie with a full paper in another journal, but he did not publish a full paper on the fermentation of the TAs after his memoir of March, 1858, to the Académie [78]. For (±)-TA, in the 1858 memoir Pasteur abandoned the name “paratartaric acid” that he had used in the earlier communication [77] and employed instead the other common name for the compound at the time, “racemic acid” (see above) [78]. It should also be noted that while the stereochemical course of Pasteur’s tartrate fermentation is described today as enantioselective, this (or any other) enantio-based term does not appear in his lectures and writings. The first enantio-based terms were introduced by Carl Friedrich Naumann (1797–1873), a German mineralogist, in 1856, but Pasteur did not adopt this terminology [79].
Pasteur’s 1858 memoir is divided into two parts [78]. Part one dealt with the fermentation of (+)-TA and Pasteur pointed out that the spontaneous fermentation of this acid had been known for a long time as a result of manufacturing accidents. He also described some of the experimental details of the fermentation as conducted in his laboratory. The fermentation mixture contained ammonium (+)-tartrate, nitrogenous “albuminoid” material from plant or animal sources, and material from a previous active fermentation of TA.
In part two the analogous incubation of (±)-TA is described. The fermentation was carried out in the same manner as that of the dextrorotatory acid, and the key experimental tool was the monitoring of the optical rotation of the mixture as the fermentation proceeded. It was found that the reaction mixture, which showed no optical rotation at first, became levorotatory as the fermentation progressed over several days. The rotation continued to increase and eventually reached a maximum, at which point the fermentation stopped. The dextrorotatory acid was no longer present in the mixture, having been destroyed in the fermentation. (−)-TA, which was not affected by the “ferment,” could then be readily isolated in pure form from the mixture. In the remainder of the memoir Pasteur proposed an explanation for the selective destruction of (+)-TA in the fermentation [76, 78] (see below).
Pasteur did not identify a specific microorganism in the memoir on the fermentation of TA [78], although he referred to the organism as “yeast.” He also described it as resembling the lactic ferment, i.e., the microorganism he had identified as responsible for lactic fermentation. In his scientific biography of Pasteur, Duclaux suggested that the microorganism of the tartrate fermentation may have been a species of Penicillium, a fungal microorganism [80]. In fact, in 1860 Pasteur reported in a brief note that Penicillium glaucum, a common mold, enantioselectively metabolized paratartaric acid in a manner very similar to the earlier fermentation: here too, (+)-TA was consumed and (−)-TA was left behind largely untouched [81].
The nature of the products of the fermentation of TA was not addressed by Pasteur in his memoir of March, 1858 [78]. He indicated in the memoir that he would soon publish information on this matter, but no such publication ever appeared. He did mention in the memoir an earlier report from the literature that identified metacetonic acid as a product of the fermentation of calcium (+)-tartrate. “Metacetonic acid” is an old name for propionic acid [82].
No indication is given in the memoir of March, 1858, whether (−)-TA was separately incubated under the conditions of the fermentation, although the brief statement in the first report (of December, 1857, [77]) suggests that such an experiment had in fact been carried out. In a related matter, in 1853 Pasteur had discovered the racemization of TA when he heated (+)-TA with a cinchona alkaloid, e.g., cinchonidine [83]. Among the products of the reaction he found not only (±)-TA but also meso-TA (Fig. 3). He recognized that this molecule, previously unknown, was inherently achiral and that therefore the substance was non-resolvable. Interestingly, however, he did not include the meso acid in the investigation of the tartrate fermentations.
Pasteur’s discovery of biological enantioselectivity in 1857 was a key finding that formed the foundation stone of the science of molecular biochirality. However, nearly 30 years elapsed after Pasteur’s discovery before the next landmark observation was made. That event and its significance will be detailed in the next section.
6 The First Finding of Enantioselectivity at a Biological Receptor
In 1886, Italian chemist Arnaldo Teofilo Pietro Piutti (1857–1928) (Fig. 4) discovered enantioselectivity in what is considered today receptor-mediated biological activity [84, 85].
Receptors are macromolecules “at the cell surface and within cells that mediate the effects of chemical messengers and hormones and the actions of many drugs in the body” [86]. The receptor concept was introduced at the dawn of the twentieth century, independently by the German physician and immunologist Paul Ehrlich (1854–1915) [87] and the British physiologist John Newport Langley (1852–1925) [88]. However, the concept of receptors was not widely accepted until the 1960s. More recently, the science of receptors has undergone an explosive growth and has assumed great importance in many areas of the biological sciences, including neuroscience, immunology, biochemistry, molecular biology, physiology, and pharmacology. Indeed, today receptors constitute one of the most intensively studied areas of biology.
Piutti completed his university education in chemistry at the University of Turin in 1879 and in 1881 moved to Florence to work with Ugo (Hugo) Schiff, an eminent professor of chemistry originally from Germany (he is mostly remembered today for “Schiff’s bases” which he discovered). It was in 1886, while working in Schiff’s laboratory, that Piutti made his discovery of enantioselectivity at receptors [85]. The discovery concerned the amino acid asparagine. In 1886 L-asparagine had already been known for 80 years. “Ordinary asparagine,” as it was often referred to, is today’s L-asparagine (Fig. 5); the latter name will be used in this chapter (and D-asparagine for its enantiomer). However, when quoting or discussing earlier writings in context, the original nomenclature (e.g., “ordinary asparagine” for L-asparagine) will be retained. The history of the first 125 years of asparagine has been described in detail by Vickery and Schmidt [89], and only a few relevant particulars will be provided here.
L-Asparagine (a non-essential amino acid) is thought to have been the first amino acid identified in natural sources and was first isolated in 1806 by the renowned French chemist and pharmacist Louis Nicolas Vauquelin (1763–1829) and his young assistant (and later a respected chemist and pharmacist in his own right) Pierre Jean Robiquet (1780–1840) [90]. They obtained the substance from the juice of the asparagus plant they indicated to be Asparagus sativus. Linn. L-Asparagine is now known to occur in the free state in many other plants as well, e.g., marshmallow, vetches, soybeans, and white lupino beans.
In the spring of 1885 Piutti assisted in the production on a large scale of ordinary asparagine in a factory producing the substance in Siena, Italy. From 6,500 kg of germinated vetch, 20 kg of crude levorotatory asparagine was obtained. The mother liquors remaining after this operation deposited, with time and natural evaporation, a mixture of two enantiomorphous crystal types, one being L-asparagine and the other a new species. Piutti mechanically separated the crystals and purified the material, obtaining in this manner 100 g of a substance whose crystals were enantiomorphous with the crystal habit of natural asparagine. The optical rotation of the new substance was found to be equal in absolute magnitude and opposite in direction to that of natural asparagine. In addition, the chemical properties and elemental composition of the new compound were the same as those of L-asparagine. No additional details on the isolation procedure are provided [84].
The chemical structure of asparagine was known in its major features at the time but not in all of its details. Specifically, the presence of the amino, carboxyl, and carboxamide groups was known, and it was understood that the latter two were separated by two saturated carbons. However, the position of the amino group was uncertain, i.e., it was not known whether the amino group is located α to the carboxyl group or α to the carboxamide function. Accordingly, Piutti asked the question whether the two asparagines (i.e., the “ordinary” form and the newly isolated compound) could be constitutional isomers, i.e., differing insofar as the position of the amino group is concerned, namely, that one of the two substances would have the structure HO2CCH(NH2)CH2CONH2 (an alpha-amino acid) while the other would correspond to HO2CCH2CH(NH2)CONH2.
In an attempt to answer this question, Piutti synthesized a series of derivatives of the two compounds and compared in each case the two analogous derivatives obtained from the two asparagines, respectively, for their chemical properties. He found no differences. However, when he compared the optical rotations of the two derivatives in each pair they were opposite in sign. He then reached the conclusion that his newly isolated compound was the “inverse” (i.e., the mirror-image form, in molecular terms) of L-asparagine [84]. Eventually Piutti settled the question of the exact structure of asparagine with an unequivocal synthesis that was imaginative and elegant for his time. He thus showed that asparagine is in fact an alpha-amino acid [91].
Even during the isolation and purification process leading to the new asparagine Piutti noticed that the mixture of the two asparagines tasted sweet. The pure D-asparagine obtained by Piutti retained the sweet taste and in this differed drastically from L-asparagine, which was without taste. Piutti stated that other known amidated acids have a sweet taste, and, importantly, he pointed out that in other known examples of enantiomerically related substances the taste does not differ [84]. Thus, examination of the taste of D-asparagine vs that of the L enantiomer was the first example of a difference in taste found for enantiomerically related substances. In hindsight it was also the discovery of the first example of enantioselectivity at a biological (human) receptor.
Given the proportion of the two asparagines Piutti isolated from the same batch of vetches (20 kg L vs 100 g D), it is logical to ask whether the D-asparagine he obtained was the result of the actual presence of the substance in the plant or whether it was an artifact produced by the partial racemization of L-asparagine during the isolation procedure. This question was raised as early as 1910 by Hans Pringsheim (1876–1940), a professor of chemistry at Berlin, who then carried out experiments in search of the answer. He boiled aqueous solutions of L-asparagine under reflux for 12–16 h and demonstrated via fractional crystallization of the material obtained that partial racemization had occurred and thereby D-asparagine produced. He therefore concluded that Piutti’s isolation of D-asparagine was the result of the partial racemization of L-asparagine during the extraction and isolation. Pringsheim also stated that no D-amino acid occurred naturally, at least based on the evidence available up to that time [92].
Piutti reacted to Pringsheim’s claim by carefully examining the occurrence of the two enantiomers of asparagine in plants. To address Pringsheim’s claims, Piutti first demonstrated that boiling aqueous solutions of L-asparagine does indeed cause, given enough time, detectable racemization. In the next step however he showed that if the temperature of the solution of L-asparagine is not allowed to rise above 55 °C no racemization takes place. He then extracted asparagine from lupines (Lupinus albus) while assuring that the temperature during the entire operation did not rise above 40 °C. Both L- and D-asparagine were found to be present in the plant extracts, proving that D-asparagine does in fact occur naturally in the plant and that his isolation of the substance was not the result of the racemization of L-asparagine. Piutti added that Pringsheim’s conclusion that no D-amino acid occurred in nature was thus unjustified [93]. According to a standard source on amino acid chemistry, Piutti’s isolation of D-asparagine in 1886 was one of the first two examples of the preparation of a D-amino acid [94].
In summary then, Piutti’s discovery of a difference in the taste of D- and L-asparagine in 1886 was a milestone first observation of enantioselectivity at a biological (human) receptor. The discovery was also the first observation of stereoselectivity of any kind in taste, the first finding of biological enantioselectivity in an organism higher than microorganisms, the first example of biological enantioselectivity in an effect other than enzyme action, and one of the two earliest reports of the preparation of a D-amino acid. Piutti also proved the structure of asparagine with an elegant synthetic pathway and showed that D-asparagine occurs naturally by demonstrating that its isolation can be carried out without any racemization of L-asparagine which is also present in the plants. Overall, Piutti’s investigations on asparagine addressed a series of challenging problems and were carried out with originality and imagination, and the result was a key discovery in the history of molecular chirality in biology. Today chiroselectivity at biological receptors is recognized as an important aspect of ligand-receptor interactions and the phenomenon has substantial implications and consequences for the science and associated technologies, e.g., in new-drug development.
7 Early Studies of Chiroselectivity in the Biological Actions and Fate of Chiral Substances
Advances in organic chemistry during the second half of the nineteenth century began the era of the elucidation of the structures of organic molecules, including many chiral molecules, and by the end of the century the two-dimensional structures (i.e., the connectivity of the atoms) of many organic compounds were elucidated. As a result of these advances, many natural or synthetic chiral compounds of known structure became available. However, elucidation of the structures of more complex molecules remained a challenge. Thus, the structures of many compounds remained unknown or were formulated incorrectly. For example, the English edition of Adolph Strecker’s Short Text-Book of Organic Chemistry, published in 1882 and authored by Johannes Wislicenus (1835–1902), a leading German chemist of the time, included many naturally occurring chiral substances, e.g., camphor, codeine, morphine, quinidine, quinine, etc., and optical rotation data were provided for many of them, but their chemical structures were not given since they were unknown.
Concerning the stereochemical aspects of chemical structures, the asymmetric carbon atom proposed in 1874 by van't Hoff and Lebel had solved the “mystery” of molecular chirality and provided an explanation for optical activity (see above). Thus, the stereochemistry could now be addressed (a good example in this regard was Piutti’s determination of the molecular structure of asparagine and his recognition of the mirror-image relationship of his new asparagine to the known “ordinary” asparagine, as discussed above). However, it should be remembered that absolute stereochemical configurations were not known at the time and comparisons and chemical correlations of chiral molecules provided only relative configurations.
As a result of the advances in organic chemistry and despite the shortcomings in molecular-structure determination mentioned above, the chemical structures of many compounds became known by the final decades of the nineteenth century which in turn led to studies of potential chiroselectivity in the biological properties of many chiral substances. Since initially only in relatively few cases were both enantiomers available separately, at first only a small number of studies of enantioselectivity appeared (some of the investigations compared the activity of one of the enantiomers to that of the racemate). With time, however, the pace accelerated, and many studies were published on the role of molecular chirality in biology. Two general areas were addressed: (1) chiroselectivity in enzymatic reactions, as manifested in the metabolic fate or enzyme-catalyzed specific transformations of substances and (2) chiroselectivity in the physiological, pharmacological, or toxicological effects of a variety of biologically active compounds.
The earliest studies investigated natural or physiological compounds, but eventually, with the advances in organic chemistry and pharmacology, synthetic substances of stereochemical interest became available and the role of chirality in the actions and disposition of such agents also began to receive attention. In this section some of the early studies of chiroselectivity are reviewed. Many of these investigations made important contributions to the budding field of the stereochemical aspects of biologically active compounds. Several reviews from that time period summarized and analyzed the early findings [95–99].
Concerning the first area of interest mentioned above, beginning at the end of the nineteenth and continuing into the first decades of the twentieth century, a great deal of work was carried out to examine the stereochemical course of enzyme-catalyzed reactions, i.e., metabolism or biochemical transformations by microorganisms, tissue preparations, crude enzyme extracts, or intact animals. Enantioselectivity was shown for many physiological compounds, e.g., amino acids, peptides, carbohydrates, lactic acid, etc., but some foreign compounds were also studied. Specific biochemical reactions were also studied, e.g., hydrolysis, oxidation, and reduction [98]. For example, pig liver esterase was found to catalyze enantioselectively the hydrolysis of mandelic acid esters [100]. These studies were carried out using the racemates, and, interestingly, when the esterase action on the separated individual enantiomers was examined, an enantiomeric interaction was revealed. Thus, when incubated separately, (−)-ethyl mandelate was hydrolyzed more rapidly than the dextrorotatory ester, while the latter was hydrolyzed faster than (−)-ethyl mandelate when the racemate was incubated [101].
Monumental work was carried out on the role of stereochemistry in biology, particularly on chiroselectivity in enzyme action (on sugars, amino acids, and peptides), by the eminent German chemist Emil Fischer (1852–1919) during ca. the last two decades of the nineteenth and early in the twentieth century. His ground-breaking work in a variety of chemical and biochemical areas has been amply chronicled [102], and only the relevant stereochemical aspects will be discussed here. Fischer began his work on carbohydrates in 1884 and pioneered the synthesis of sugars, including stereoisomeric forms, and the determination of their structures and stereochemical relationships. Indeed, Fischer’s work on the synthesis of sugars qualifies him as a pioneer of asymmetric synthesis [103, 104] Fischer applied the fundamental concepts of van't Hoff and Lebel concerning the asymmetric carbon atom and its role in stereochemistry, and his work in this regard was a powerful validation of the ideas of the two chemists. Fischer’s work also benefited from the great advances in structural organic chemistry, valence theory, etc., achieved during the second half of the nineteenth century.
Fischer studied both natural and synthetic sugars, and demonstrated that the microbial fermentation (e.g., by beer yeast) and other enzymatic reactions of sugars displayed considerable stereoselectivity. He found both diastereoselectivity and enantioselectivity in the action of enzymes on sugars. For example, he showed that natural glucose, fructose, and galactose were readily fermented by yeast but their “optical antipodes” (i.e., the respective enantiomers) were left unchanged [105]. As for diastereoselectivity, he found, for example, that crude aqueous yeast extracts (which he named “invertin”) hydrolyzed α-methyl-D-glucoside but not its diastereoisomer (epimer) β-methyl-D-glucoside, while a preparation obtained from almonds Fischer called “emulsin” hydrolyzed β-methyl-D-glucoside but not α-methyl-D-glucoside (the methyl-L-glucosides were entirely unaffected) [105]. Fischer also realized that “fermentation” by microorganisms is almost certainly the result of the action of enzymes within them, and his findings on the stereoselectivity of enzyme action prompted him to propose a model to rationalize the observations (his model will be discussed below, together with other early proposals to explain biological chiroselectivity). Fischer also made an important contribution to stereochemistry with his system of drawing stereochemically explicit and convenient structures using a convention now known as the Fischer projection [106].
Fischer was intrigued by nature’s ability to synthesize sugars in unichiral form, and, as pointed out by Ramberg in his insightful analysis of Fischer’s work on sugars, Fischer concluded that “asymmetric” chemical constituents within cells were responsible for this asymmetric synthesis, rather than external universal “dissymmetric forces” (e.g., sunlight or magnetism) believed by Pasteur to be the agents ultimately responsible for the unichiral character of natural compounds [107].
Today it is generally agreed that Fischer’s work was revolutionary. His groundbreaking work was recognized in 1902 with the Nobel Prize in chemistry for “the extraordinary services he has rendered by his work on sugar and purine syntheses.” In the award address, Professor Hj. Théel, President of the Swedish Royal Academy of Sciences, specifically discussed the revolutionary importance of Fischer’s work on sugars, their stereochemistry, and the nature of enzymes and their interactions with sugars, and emphasized the overall impact of Fischer’s work on the essential connections of chemistry and biology [108].
As attention began to be focused on enantioselectivity in biology at the end of the nineteenth century, metabolism in organisms higher than microorganisms was also investigated. For example, when racemic camphor was fed to dogs or rabbits, more of the levo enantiomer was converted to a glucuronyl conjugate than of the dextro enantiomer, and when (±)-malic acid was injected subcutaneously into rabbits, larger amounts of the unchanged dextrorotatory acid appeared in the urine, indicating that (−)-malic acid, the naturally occurring form, was more extensively metabolized; other compounds were also studied [109].
A complex picture of stereoselectivity emerged from the investigations of metabolic fate or enzymatic transformations, depending on the substrate, the microorganism, plant, animal species, or particular enzyme involved, and the biochemical reactions catalyzed. In some cases no enantioselectivity was found while in others only one of the enantiomers was acted upon; in some cases both enantiomers functioned as substrates for an enzyme (or were destroyed in vivo) but at different rates. Furthermore, in some cases the direction of enantioselectivity changed for the same substrate with the microorganisms or enzymes used. However, interpretation of the results of these studies is at times complicated by the fact that the nomenclature used was, in hindsight, confusing. The d and l descriptors, for example, were originally introduced to indicate configuration, and later were used to refer to optical rotation [110], and this dual use renders the interpretation at times difficult indeed. To illustrate, Cushny wrote: “d-glucose is dextrorotatory…while d-fructose is laevorotatory.” On the same page he also wrote: “in the same way a l-rotary [sic] substance may be destroyed by one enzyme in preference to the d-rotary isomer…” [111]. The potential confusion is obvious.
The other major area of interest, namely biological activity other than enzyme action, was also studied. As discussed above, the first observation of enantioselectivity in such activity was Piutti’s finding of a difference in the taste of the asparagine enantiomers. Piutti’s discovery prompted similar studies by others, and the taste of several amino acids was examined shortly thereafter. For example, in 1894 Menozzi and Appiani reported that the enantiomers of glutamic acid differed in their taste. The authors pointed out that their finding was not new, inasmuch as a difference in the taste of the enantiomers of asparagine had already been reported by Piutti [112]. Beginning during the end of the nineteenth century, many studies comparing the enantiomers of substances of pharmacological or toxicological interest were carried out, and many examples of enantioselective effects were observed.
The first clear example of enantioselectivity in a pharmacological action proper was the demonstration by Cushny that (−)-hyoscyamine was ca. 12–20 times more potent than the dextro enantiomer in a variety of pharmacological effects, e.g., mydriasis in the cat, salivary secretion in the dog, and at cardiac myoneural junctions. Interestingly, (+)-hyoscyamine was the more potent enantiomer in CNS-excitatory effects [113]. Cushny also found that the enantiomers of epinephrine (adrenalin) differ significantly in their ability to increase blood pressure, the levorotatory form being 12–15 times more potent than (+)-(S)-epinephrine [114]. Arthur Robertson Cushny (1866–1926), a Scottish pharmacologist, made important experimental contributions to the field of enantioselectivity in pharmacology; in 1926 he reviewed the studies of enantioselective pharmacology and enzyme action published during the previous ca. 40 years [115] and provided a detailed and critical discussion of enantioselectivity in biology that revealed a great deal of insight into the nature of chirality and its biological implications. Cushny was a leading figure in the discovery of the role of chirality in biology, particularly in pharmacology.
An interesting, complex, and important example of biological diastereoselectivity (with implications for enantioselectivity) between chiral molecules concerns the natural unichiral substances quinine and quinidine (Fig. 6), two important cinchona alkaloids (some aspects of the history and antimalarial, antiplasmodial effects of quinine were discussed in an earlier section of this chapter). Natural quinine is levorotatory and quinidine dextrorotatory.
The two alkaloids are diastereoisomerically related and differ in configuration at two of the five stereogenic centers. Thus, in quinine C8 and C9 are S and R, respectively, while in quinidine they are reversed, i.e., R and S, respectively (Fig. 6). (The literature shows some confusion concerning the stereochemical relationship of the two substances, with claims by some authors that the two substances are related as enantiomers [116].) In 1853 Pasteur recognized that quinine and quinidine were “isomers” but not enantiomers, and studied the optical rotation, crystallographic properties, and some chemical reactions of the two substances [117]. However, the structures of quinine and quinidine were then unknown.
Today the main therapeutic use of quinine is as an antimalarial (other uses include the treatment of nocturnal leg cramps and application as a flavoring agent in food). Interestingly, quinidine is somewhat more potent as an antimalarial (against Plasmodium falciparum) than quinine and is used in that indication in some clinical settings [118]. Epiquinine and epiquinidine are the epimers at (the hydroxyl-bearing center) C9 of quinine and quinidine, respectively, with the configurations at C8 and C9 being R,R in epiquinidine and S,S in epiquinine. Epiquinine and epiquinidine, which are also present in cinchona, are essentially devoid of antimalarial activity, i.e., the antiplasmodial action is lost when the configuration at the C9 center only is inverted in quinine or quinidine [118]. It is clear therefore that the antimalarial activity requires the R,S/S,R relative configuration at C8,C9. Potential explanations of this radical change in biological properties produced by configurational change at C9, i.e., at only one of five stereogenic centers, are naturally of interest. Attempts have been made to discern conformational or physicochemical factors that may account for the difference between the antimalarial potencies of the two sets of alkaloids (i.e., quinine/quinidine vs epiquinine/epiquinidine). For example, a recent theoretical study of the conformational spaces of the cinchona alkaloids using a semiempirical method found significant conformational differences between the quinine/quinidine set on the one hand and the epiquinine/epiquinidine set on the other. Furthermore, it was also found that the conformational spaces of epiquinine and epiquinidine feature important conformers with an intramolecular hydrogen bond that is absent in quinine and quinidine [119]. It remains to be determined whether these conformational factors play a role in the profound difference in antimalarial activity between the two epimerically related sets of substances.
Quinidine has been mainly employed as a cardiac antiarrhythmic agent. The antiarrhythmic use of cinchona alkaloids goes back to the eighteenth century, when extracts of the cinchona bark were employed in the treatment of “rebellious palpitations” [120], and by the early 1920s quinidine was in clinical use as an antiarrhythmic agent. In 1922 Deschamps described his extensive studies comparing the cardiovascular properties and clinical effects of quinidine and quinine, and also gave a detailed review of previously published cardiovascular investigations of the two drugs [121]. Deschamps concluded that the two substances are qualitatively similar in their cardiovascular effects but quinidine is considerably more potent. He also indicated that, of the two drugs, only quinidine was clinically useful as an antiarrhythmic agent since the therapeutic effects of quinine are weak and variable. Deschamps’ analysis has been validated by the subsequent experience, and quinine is in fact not used as an antiarrhythmic drug. The difference between quinine and quinidine in this regard is a notable example of receptor-mediated chiroselectivity between diastereoisomers.
As indicated above, the configurations of the C8 and C9 centers in quinine are the opposite of those in quinidine, and in some systems the two compounds behave as if they were enantiomerically related, prompting some investigators to refer to the two molecules as “pseudoenantiomers” [119, 122]. In this light, it is of interest to recall that both quinine and quinidine are potent antimalarial agents, i.e., their “pseudoenantiomer” relationship does not result in a large difference in antiplasmodial potency. In antiarrhythmic effects and some other properties [123, 124], on the other hand, the two drugs do display considerable differences. These observations suggest that investigations of enantioselectivity in the biological actions of quinine and of quinidine may be instructive. However, the respective enantiomers of natural quinine and quinidine have not been described in the literature. Clearly, additional studies are needed to reveal the molecular, physiological, and pharmacological bases of the stereoselectivity in the actions of the cinchona alkaloids.
An important group of synthetic drugs is that of the barbituric acid derivatives. Such drugs (“the barbiturates”) were introduced into pharmacotherapy as sedatives, hypnotics, anesthetics, or anticonvulsants beginning early in the twentieth century (e.g., phenobarbital, anticonvulsant; preparation patent 1911). Some of the early barbiturates were chiral and several of them are still in use (as racemates) in clinical medicine today, e.g., pentobarbital (sedative, hypnotic, anesthetic; preparation patent 1916), mephobarbital (anticonvulsant; preparation patent 1929), thiopental (ultra-short-acting anesthetic; preparation patent 1939), etc. The earliest investigation of enantioselectivity in the biological effects of a barbiturate appeared in 1928 and examined the properties of the enantiomers of 5-ethyl-5-α-methylheptylbarbituric acid [125]. The compounds were tested in albino rats, and no significant differences were found between the enantiomers in their hypnotic efficacy and acute toxicity. These results prompted the authors of the study to reaffirm the validity of the then prevailing view that anesthetic action is correlated with the lipophilicity (lipid solubility) of the drugs and is not influenced by their specific chemical-structural details, a view embodied in the Meyer–Overton theory [126]. Since the two enantiomers of a chiral substance do not differ in their lipid solubility (in an achiral environment), a lack of enantioselectivity would in fact be expected for the barbiturates.
However, it is now known that a number of chiral anesthetics, including several barbiturates, show significant enantioselectivity in their anesthetic and other effects [127, 128], a phenomenon which is incompatible with the Meyer–Overton correlation. It has in fact become clear in recent decades that anesthetic agents produce anesthesia via interaction with specific receptors (proteins) such as ion channels [129], and therefore enantioselectivity is not unexpected for these drugs.
In a recent attempt to account for the lack of enantioselectivity in anesthetic effect observed in the 1928 study of 5-ethyl-5-α-methylheptylbarbituric acid, it was suggested that a possible explanation may be based on Pfeiffer’s rule [130]. In 1956 Pfeiffer found a correlation between the average effective human dose of a series of racemic drugs and the ratio of the potencies of the two enantiomers of each drug [131]. It was found that the log values of the ratio of potency of the enantiomers plotted against the log of effective human dose of the racemate gave a linear relationship; as the effective racemic dose increased the ratio of the efficacy of the enantiomers decreased. Surprisingly, the correlation held for a variety of drugs, regardless of pharmacological class or effect, pharmacokinetic differences, etc. The interpretation offered was that when the effective dose is small, the affinity of the more active enantiomer for the receptor is high, which in turn means a tighter binding to the receptor, i.e., greater steric constraints in the binding of the drug to the receptor. Such steric constraints (tight binding) would of course magnify the difference between the enantiomers since the “wrong” enantiomer would have a lesser ability to bind productively to the receptor due to the tighter steric requirements. Therefore, it has been argued on the basis of Pfeiffer’s rule that since the effective anesthetic dose of the racemic barbiturate (5-ethyl-5-α-methylheptylbarbituric acid) used in the 1928 study is particularly large (360 mg/kg) when compared to many other barbiturates, the anesthetic effect is in fact expected to display little if any enantioselectivity [131].
The complexities and limitations of Pfeiffer’s rule have been discussed [132], but there is a puzzling detail in Pfeiffer’s article that does not appear to have caught the attention of commentators. The issue is the fact that among the drugs included by Pfeiffer in the correlation were quinine and quinidine. As mentioned above, the respective enantiomers of natural quinine and quinidine have not been described in the literature, and therefore the ratios of the potency of the enantiomers for the two drugs could not have been available to Pfeiffer. Moreover, neither racemic quinine nor racemic quinidine has been used as a human drug. It is thus puzzling indeed that Pfeiffer included the two drugs in his correlation, since the data on these drugs needed for the correlation (i.e., the effective human doses of the two racemates and the ratios of the potencies of the enantiomers) were not available to him.
As the early examples of biological chiroselectivity (particularly enantioselectivity) began to unfold (as outlined above), it was to be expected that mechanistic explanations for the phenomenon would be sought. The first such efforts and proposed models will be described in the next section.
8 First Explanations of Biological Enantioselectivity
The first proposal of an explanation for the existence of biological enantioselectivity was provided by Pasteur himself in conjunction with his discovery (see above) of the enantioselective fermentation of TA by a microorganism [78]. He proposed that a unichiral substance within the constitution of the microorganism is involved in the utilization of the tartrate molecules as nutrients, and stated the fundamental principle that two enantiomerically related molecules (the tartrate enantiomers in this case) interact differently (i.e., form different “combinations”) with a third chiral molecule, i.e., a unichiral constituent of the microorganism, and explained that the latter does not “accommodate” equally well the left- and right-tartrate molecules. He recognized that the two “combinations” no longer had an enantiomeric relationship. This difference between the two complexes, he added, allows for different properties and behavior by the two combinations, i.e., the possibility of differences in biological effects by the two enantiomers [78].
It is noteworthy that Pasteur’s fundamental explanation of enantioselective biological action is still considered valid today. The two complexes are indeed not related as enantiomers, and can therefore have different properties, i.e., the two enantiomers can produce different biological effects. Pasteur advanced the idea that a diastereomeric relationship (by today’s terminology) between the ligand-mediator complexes exists and explains the discrimination between the enantiomers. Given that almost nothing was known at the time about organic chemical structure, enzymes, or microbial function, Pasteur’s explanation was indeed far ahead of its time. He understood the fundamental stereochemical phenomenon that leads to biological enantioselectivity, and his words, “we see here the property of molecular dissymmetry possessed by natural materials intervening in a physiological phenomenon as a modifier of affinity,” show a clear recognition of the essence of chirality as a modulator of molecular recognition in biology, as we would put it today.
The next proposal attempting to explain the chiroselectivity seen in the interaction of chiral molecules with biological systems came from Fischer and was the result of his extensive studies of enzymatic reactions of sugars and peptides using microorganisms or crude enzyme preparations. Fischer concluded that overall shape and stereochemical configuration strongly influence the suitability of a molecule to serve as substrate for an enzyme. He condensed these spatial requirements in the statement that for an enzyme to act on a substrate the two must fit like a lock and its key. Fischer used this metaphor (“Schlüssel-Schloss-Prinzip” in German) for the first time in a paper that appeared in 1894 [105]. Although the three-dimensional structure of enzymes was unknown at the time, their role as protein-like substances became quite apparent. In a comprehensive article on the significance of stereochemistry to physiology that appeared in 1898, Fischer summarized the basis of his analogy: “Although one does not know these substances [enzymes] in a pure state, their similarity with proteins is so close and their generation from these so probable, that they have undoubtedly to be considered as optically active, and, hence, asymmetric molecular forms. This had led to the hypothesis that there must be a similarity in the molecular configuration between the enzymes and their object of attack, if reaction is to take place. To make this thought more perspicuous, I have used the picture of lock and key” [99].
Fischer’s model has become an enduring paradigm in enzyme-substrate interactions. Furthermore, the model clearly has a certain broader generality for interactions of molecules with biological systems in that it states simply that, for productive contact, the two interacting components must fit according to some structural constraints. The concept is indeed broad, and not surprisingly therefore it has served as a rationale to account for complementarity in biological responses in contexts other than stereochemistry. For example, Paul Ehrlich (see above) harnessed the argument as a theoretical basis for his side chain theory [133]. To explain the immunological response, Ehrlich postulated that one of the side chains (or receptors, as he coined later) of the cell has an atomic grouping with a specific combining property for a toxin. He also distinguished between the so-called toxophore and haptophore groups of the toxin, the latter being the atomic group involved in binding the toxin to the side chain. As Ehrlich wrote in a 1900 publication, each cell possesses a number of side chains, which bind toxins in a lock-and-key type manner [87]. Furthermore, American zoologist Frank R. Lillie (1870–1947), a pioneer of embryology, invoked the lock-and-key analogy to describe the interaction between sperm and the egg receptor as a preliminary step in his theory of fertilization [134]. Overall, it appears clear today that on introducing the lock-and-key model for enzyme-substrate interactions, Fischer anticipated the field of molecular recognition and contributed to the intimate linkage of synthetic chemistry and biology, in the modern sense of interdisciplinary science.
It should be noted, however, that Fischer’s conception of the lock-and-key model has also received some criticism. For example, Fruton [135] interpreted Fischer’s view of his lock-and-key model as based on what Fischer believed to be the chemical similarity between the enzymes and their substrates, rather than on the complementarity between the interacting structures, the latter view being held today as the basis of phenomena of molecular recognition of the type Fischer encountered. However, Fischer’s statement that “there must be a similarity in the molecular configuration between the enzymes and their object of attack” (see above) does not stress chemical similarity per se and focuses on similarity in “molecular configuration,” i.e., the geometric aspects rather than chemical similarity.
A strong criticism of Fischer’s lock-and-key paradigm came from Fajans, who in 1910 discussed Fischer’s model and argued that it was too restrictive inasmuch as it held that one “antipode” of a chiral substrate fits the enzyme and is therefore acted upon by the enzyme, while its enantiomer does not fit and is accordingly left unaltered [95]. Fajans pointed out in contrast that considerable variability in enzymatic enantioselectivity had been reported in the literature, including many cases where both enantiomers of a substance serve as substrates for a given enzyme (albeit often the reaction rates differ). Fajans interpreted this state of affairs as incompatible with the lock-and-key model, pointing out that a key would not fit the mirror-image version of its lock, while an enzyme often catalyzes the reaction of both enantiomers as substrates [95]. However, Fajans’ critique of Fischer’s lock-and-key metaphor has not received significant support in the literature.
As we have seen, Pasteur’s model focused on enantioselectivity in biology while Fischer’s proposal had a broader character and included diastereoselectivity. However, neither Pasteur’s nor Fischer’s model invoked any specific molecular details or rules. The first specific chemical-structure-based proposal explaining enantioselective biological effects was published in 1933 by Easson and Stedman [136]. They proposed a model for the differential binding of (−)- and (+)-epinephrine (Fig. 7) at the “adrenergic receptor” (the existence of multiple types of adrenergic receptors was unknown at the time) as the explanation for the considerable difference between the enantiomers in raising blood pressure. As mentioned above, the levorotatory enantiomer [(−)-(R)-epinephrine] had been shown to be ca. 12–15 times as potent as dextro-epinephrine [114].
The model advanced by Easson and Stedman was based on a paradigm of three points of interaction between the ligand and the receptor. It was proposed that the amino functionality, the benzylic hydroxyl group, and the electron-rich aromatic ring of epinephrine (Fig. 7) are groups that can interact with three respective complementary moieties strategically located on the (chiral) receptor. If one of the epinephrine enantiomers (i.e., levo form in this example) is able to bind fully with all three of its groups to the three complementary groups on the receptor (and thereby produce the full biological effect), then it can be readily demonstrated that dextro-epinephrine cannot interact fully in the same manner and at best only two of its three groups can bind simultaneously to their two respective complementary sites on the receptor. A two-point attachment means weaker binding than the full, three-point, connection, and this in turn produces a weaker biological effect. Such weaker binding would then account for the difference between the two epinephrine enantiomers in their pressor effects [136].
In support of this argument Easson and Stedman pointed out that if the benzylic hydroxyl group of epinephrine is replaced with a hydrogen atom, the resulting (achiral) molecule (epinine) would bind to the receptor in the same manner as (+)-epinephrine, i.e., only at two points (since in epinine one of the three binding groups, the benzylic hydroxyl, is no longer present). Therefore, epinine’s effect on blood pressure should be weaker than that of (−)-epinephrine, i.e., epinine should be similar to (+)-epinephrine in its potency, a prediction borne out by the facts.
The differential attachment of the enantiomers to the receptor in this model is the result of simple three-dimensional-geometry considerations: two three-dimensional enantiomorphous structures (the epinephrine enantiomers here) in principle cannot attach in an identical manner to a third chiral structure (the receptor) if there are at least three points of binding (the fundamental assumption in this basic analysis is that the binding or attachment points are the only “interactions” between the two structures). This in turn implies that in three-dimensional space a minimum of three interaction points are required for potential enantioselectivity. Thus, fundamentally, the three-point-interaction model is not a biological concept but a geometry-dictated paradigm. Indeed, the model has been successfully applied in non-biological contexts, e.g., to account for enantioselective chromatographic separations [137].
The three-point-interaction model is of course also applicable in biology, as in the present context to ligand-receptor binding, and can be extended to account for biological enantioselectivity in general. Such generalized adoption of the model has in fact spread in discussions and considerations of biological enantioselectivity. However, it is important to recognize that the theoretical three-point-interaction model is the minimal geometric paradigm and does not preclude the occurrence of ligand-to-mediator binding at more than three points (and associated enantioselective effects) in any given case – it simply states that, due to fundamental geometry principles, in three-dimensional space a minimum three points of interaction are required for potential enantioselectivity to occur.
In 1935 Bergmann et al. postulated three points of contact for enantioselective enzymatic reactions [138] but did not cite the earlier proposal by Easson and Stedman. In 1948 the three-point-interaction model was again proposed, this time by Ogston, in the context of prochirality in enzymatic reactions [139]. The issue concerned the tricarboxylic-acid (TCA, citric-acid, or Krebs) cycle in intermediary metabolism, and Ogston proposed the three-point-interaction model to explain the ability of one of the enzymes in the cycle to distinguish between two identical groups within the substrate, citric acid, an achiral molecule. The “identical” groups were the two –CH2COOH groups of citric acid. The stereochemical result of the reaction of citric acid catalyzed by the enzyme had confused biochemists, and Ogston’s explanation using the three-point-attachment paradigm between the achiral substrate and the enzyme provided the answer to the stereochemical “puzzle” [139]. Today the two “identical” –CH2COOH groups (ligands) of citric acid are described as “enantiotopic,” a term that is defined as referring to two groups “in constitutionally equivalent locations that are related by a symmetry plane (or center or alternating axis of symmetry) but not by a (simple) symmetry axis. Replacement of one or the other enantiotopic ligand by a new ligand produces enantiomers” [140]. In its essence, Ogston’s three-point-interaction paradigm for the recognition of enantiotopic groups by an enzyme is equivalent to the three-point model proposed earlier by Easson and Stedman as an explanation of enantioselective binding by chiral molecules at receptors. Ogston cited neither the 1933 proposal by Easson and Stedman nor the 1935 article by Bergmann et al. on the three-point-attachment paradigm.
Some of the successes and limitations of the Easson–Stedman model have been discussed [141]. Although the model has been useful in accounting for the biological enantioselectivity of a variety of compounds, other studies [2] have shown that in certain cases the model needs refinements and additional elements. It has also been shown, not surprisingly, that in some systems that display enantioselectivity, more than three points of interaction can be demonstrated [142]. Some authors [143, 144] have questioned the fundamental validity of the three-point-attachment model, but such views have not received a great deal of support in the literature.
9 Final Reflections
The discovery of molecular chirality in the middle of the nineteenth century represents a watershed in the life sciences. The manual separation of enantiomorphous tartrate crystals by Pasteur has often been described as one of the most beautiful experiments in chemistry. In the subsequent ca. 100 years important discoveries concerning the role of molecular chirality in biology were made by, among others, Pasteur, Piutti, Fischer, Cushny, Easson and Stedman, etc., and their contributions paved the way to our modern understanding of chiroselectivity in biology and the intimate relationship between structure and function.
By the dawn of the twentieth century, studies of the stereochemical aspects of the biological activity, metabolism, or enzymatic transformations of natural or synthetic substances were proliferating and chiroselectivity was often found in these investigations. Overall, then, by the end of the 1930s a great deal of information on chiroselective biological phenomena had been amassed and the recognition had emerged that chiroselectivity is a common and important phenomenon in the interaction of chiral molecules with biological systems.
While this new knowledge on the biological role of molecular chirality was an enormous advance, it should also be pointed out that the information accumulated by the 1930s was not rapidly exploited for some of its possible benefits. In particular, the new understanding of the role of chirality in biology did not make an impact in the development and marketing of new chiral drugs for many decades after the 1930s. Indeed, during a long period the pharmaceutical industry and governmental drug-regulatory authorities largely ignored the implications and potential of molecular chirality for better and safer drugs. This neglect may have had its explanation, at least in part, in the lack of availability, certainly during the early part of the period in question, of scientific and technical tools essential for the research and development work needed to translate the accumulated scientific information into better and safer drugs (see below).
Be that as it may, it was only in the 1990s that this situation began to change. Thus, in 1992 the US FDA finally issued guidelines concerning the development of new drugs based on chiral molecules [145], and in the same time-frame other drug-regulatory authorities around the world also began to address the issue. The new regulations thus introduced required that during the development of new drugs the role and implications of molecular chirality in biology be taken into account. The impact of this change in the approach to chirality in new-drug development has been dramatic. For example, today the appearance of a new racemic drug on the market is a highly unlikely event (the significance of this change can be appreciated when we consider that at the time of the publication of the new FDA rules, in 1992, ca. 25% of all marketed drugs were racemic). The marketing of a racemic drug is justified in those cases where the drug is stereochemically labile, i.e., if there is in vitro or in vivo racemization or an in vivo stereochemical inversion on a time scale sufficiently fast to be relevant to the shelf-life of the drug or to its composition during the therapeutic treatment. A class of drugs where in vivo inversion of one of the enantiomers is commonly seen is the “profens,” i.e., the 2-arylproprionic-acid-based analgesic and anti-inflammatory agents [146].
Thus, in the 1990s, regulatory authorities and the pharmaceutical industry began to recognize that in many cases the racemic mixture is a combination of two biologically distinctly different substances. In most cases, in fact, the two enantiomers will differ in some aspects of their effects, and there are many examples where the enantiomers have been found to differ considerably – and at times drastically – in their pharmacology, toxicology, clinical efficacy, and/or pharmacokinetics, etc. Overall, it is generally believed today that a unichiral version of a therapeutic agent is likely to have advantages over the racemic or some other stereoisomeric mixture. For example, the pharmacology and toxicology profiles will be clearer for the single substance, the pharmacokinetics and the relationship between serum concentration and biological effects will be more readily interpretable, in some cases adverse effects or toxicity will be eliminated with the removal of the less-favorable stereoisomer(s), the dosage of the unichiral drug may be lower than that of the stereoisomer mixture, etc. A good example here is the case of racemic bupivacaine, an anesthetic agent. Both enantiomers possess anesthetic properties, but it has been demonstrated that levobupivacaine [(2S)-1-butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide] is a safer drug than the racemate (bupivacaine) due to the significant cardiotoxicity of the dextrorotatory enantiomer. As a result, levobupivacaine has been introduced as a drug with a better safety profile in anesthetic clinical practice in some countries [147].
However, a word of caution is in order here. In a few cases it has been shown that the racemic or some other mixture of the stereoisomers was a safer drug than a unichiral form [148]. Thus, each case should be considered on its own merits.
The question may be asked: what were the factors that finally produced, after a ca. 60-year delay, this change in attitude toward the role of chirality in new-drug development? In retrospect, it appears that advances in two chemical disciplines played a major role in focusing the attention on the importance of molecular chirality in new-drug development:
-
1.
The considerable advances in stereoselective synthesis during the last several decades of the twentieth century. Such improvements in organic synthesis have allowed the preparation of a wide variety of unichiral drugs, drug metabolites, and related substances, including many with highly complex stereochemistry.
-
2.
The advent, during the same period, of powerful methods for enantioselective analysis, i.e., the detection and quantification of enantiomerically related substances in the presence of each other, particularly via enantioselective chromatography. The benefits of such analytical methodology are multifold. For example, the new chromatographic analytical methods have allowed the convenient, rapid, precise, and accurate determination of the enantiomer composition or enantiopurity of chiral substances, even in cases of trace contamination of a unichiral compound with the enantiomer. Such enantioselective analytical capability is essential in the preparation of unichiral substances of high stereochemical purity and in the development of the necessary stereoselective synthetic methods; such methods are also required to assure stereochemical purity during pharmacological testing, where even low levels of contamination of the distomer (the biologically less potent enantioform) with the eutomer (more potent enantiomer) may distort the results (see Sect. 2 for a discussion on propranolol); and, of course, the new analytical methodology has also allowed the study of enantioselectivity in the pharmacokinetics and metabolism of chiral substances. (An important additional advantage of some of the enantioselective chromatographic separations is that in the preparative mode they may rapidly provide sufficient amounts of the individual enantiomers for prompt initial pharmacological and toxicological evaluation, without the need to resort to enantioselective syntheses, whose development is often challenging and time-consuming.)
In conclusion, it is clear that a strong component of the interest in the role of molecular chirality in biology derives from the implications of the phenomenon for the development of new drugs and the corresponding needs of the pharmaceutical industry. However, in a more general sense, the often-seen distinctive properties of enantiomers at a biological level have fine-tuned key mechanisms, such as replication, transcription, biosynthesis, biological activity, metabolic fate, etc., in living organisms and produce profound consequences. All in all, it is widely recognized today that molecular chirality is a fundamentally important modulator of the effects and properties of chiral substances in a variety of branches of biology and medicine.
References
Simonyi M (1984) On chiral drug action. Med Res Rev 4:359–413
Patel BK, Hutt AJ (2004) Stereoselectivity in drug action and disposition: an overview. In: Reddy IK, Mehvar R (eds) Chirality in drug design and development. Marcel Dekker, New York
Crossley R (1992) The relevance of chirality to the study of biological activity. Tetrahedron 48:8155–8178
Guijarro A, Yus M (2009) The origins of chirality in the molecules of life. Royal Society of Chemistry, Cambridge
Brocks DR (2006) Drug disposition in three dimensions: an update on stereoselectivity in pharmacokinetics. Biopharm Drug Dispos 27:387–406
Cintas P (2007) Tracing the origins and evolution of chirality and handedness in chemical language. Angew Chem Int Ed 46:4016–4024
Cintas P (2004) The road to chemical names and eponyms: discovery, priority, and credit. Angew Chem Int Ed 43:5888–5894
Gal J (2011) Louis Pasteur, language, and molecular chirality–I. Background and dissymmetry. Chirality 23:1–16
Gal J (2011) Stereochemical vocabulary for structures that are chiral but not asymmetric: history, analysis, and proposal for a rational terminology. Chirality 23:647–659
Eliel EL (1997) Infelicitous stereochemical nomenclature. Chirality 9:428–430
Helmchen G (1997) Glossary of problematic terms in organic stereochemistry. Enantiomer 2:315–318
Eliel EL, Wilen SH (1994) Stereochemistry of organic compounds. Wiley, New York, p 1194
Mislow K (1999) Molecular chirality. Top Stereochem 22:1–82
Gal J (1998) Problems of stereochemical nomenclature and terminology. 1. The homochiral controversy. Its nature, origins, and a proposed solution. Enantiomer 3:263–273
Lee ED, Henion JD, Brunner CA, Wainer IW, Doyle TD, Gal J (1986) High-performance liquid-chromatographic chiral stationary phase-separation with filament on thermospray mass spectrometric identification of the enantiomer contaminant (S)-(+)-methamphetamine. Anal Chem 58:1349–1352
Kelvin L (1894) The molecular tactics of a crystal. The 2nd Robert Boyle lecture. J Oxford Univ Junior Sci Club 18:25
Kuhnert N, Le-Gresley A, Nicolau DC, Lopez-Periago A (2007) Synthesis, self-association and chiroselectivity of isotopically labeled trianglamine macrocycles in the ion trap mass spectrometer. J Label Compd Radiopharm 50:1215–1223
Wagner N, Rubinov B, Ashkenasy G (2011) β-Sheet-induced chirogenesis in polymerization of oligopeptides. Chemphyschem 12:2771–2780
Gal J (2006) Chiral drugs from a historical point of view. In: Francotte E, Lindner W (eds) Chirality in drug research. Wiley-VCH, Weinheim, pp 3–26
Finefield JM, Sherman DH, Kreitman M, Williams RM (2012) Enantiomeric natural products: occurrence and biogenesis. Angew Chem Int Ed 51:4802–4836
Burger A (1980) Introduction: history and economics of medicinal chemistry. In: Wolff ME (ed) Burger’s medicinal chemistry, part I, 4th edn. Wiley, New York, pp 7–22
Burger A (1986) Drugs and people: Medications, their history and origins, and the way they act. University Press of Virginia, Charlottesville, p 4
Burger A (1986) Drugs and people: medications, their history and origins, and the way they act. University Press of Virginia, Charlottesville, pp 4–5
Burger A (1980) Introduction: history and economics of medicinal chemistry. In: Wolff ME (ed) Burger’s medicinal chemistry, part I, 4th edn. Wiley, New York, p 8
Chen KK, Schmidt CF (1930) Ephedrine and related substances. Medicine (Baltimore, MD) 9:1–117
Hoffman BB (2001) Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: Hardman JG, Limbird LE, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, p 237
Newton GD, Pray WS, Popovich NG (2004) New OTC drugs and devices 2003: a selective review. J Am Pharm Assoc 44:211–225
Perrine DM (1996) The chemistry of mind-altering drugs – history, pharmacology, and cultural context. American Chemical Society, Washington, pp 44–45
Foye WO (1995) Medicinals of plant origins: historical aspects. In: Foye WO, Lemke TL, Williams DA (eds) Principles of medicinal chemistry, 4th edn. Lippincott Williams and Wilkins, Baltimore, p 8
Burger A (1986) Drugs and people: medications, their history and origins, and the way they act. University Press of Virginia, Charlottesville, p 15
Burger A (1980) Introduction: history and economics of medicinal chemistry. In: Wolff ME (ed) Burger’s medicinal chemistry, part I, 4th edn. Wiley, New York, p 3
Sertürner FWA (1817) Ueber das Morphium, eine neue salzfähige Grundlage, und die Mekonsäure, als Hauptbestandtheile des Opiums. Ann Physik 55:56–89
Gulland JM, Robinson R (1923) Morphine group. I. Discussion of the constitutional formula. J Chem Soc 123:980–998
Johnson MR (1980) Analgetics. In: Wolff ME (ed) Burger’s medicinal chemistry, part I, 4th edn. Wiley, New York, p 707
Phillips J (1912) Prevalence of the heroin habit: especially the use of the drug by snuffing. JAMA LIX:2146–2147
Johnson MR (1980) Analgetics. In: Wolff ME (ed) Burger’s medicinal chemistry, part I, 4th edn. Wiley, New York, pp 708–709, 735
Eap CP, Finkbeiner T, Gastpar M, Scherbaum N, Powell K, Baumann P (1996) Replacement of (R)-methadone by a double dose of (R, S)-methadone in addicts: interindividual variability of the (R, S)-ratios and evidence of adaptive changes in methadone pharmacokinetics. Eur J Clin Pharmacol 50:385–389
Honigsbaum M (2001) The fever trail. In search of the cure for malaria. Farrar, Straus, and Giroux, New York, pp 24–25
Pelletier PJ, Caventou JB (1820) Suite: Des recherches chimiques sur les quinquinas. Ann Chim Phys 15:337–365
Honigsbaum M (2001) The fever trail. In search of the cure for malaria. Farrar, Straus, and Giroux, New York, pp ix–x
Honigsbaum M (2001) The fever trail. In search of the cure for malaria. Farrar, Straus, and Giroux, New York, pp x, xiii
Garfield S (2000) Mauve: how one man invented a color that changed the world. W. W. Norton & Co, New York
Tracy JW, Jr Webster LT (2001) Chemotherapy of parasitic infections. In: Hardman JG, Limbird LE, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, p 1069
Seeman JI (2007) The Woodward-Doering/Rabe-Kindler total synthesis of quinine: setting the record straight. Angew Chem Int Ed 46:1378–1413
Woodward RB, Doering WE (1945) The total synthesis of quinine. J Am Chem Soc 67:860–865
Stork G, Niu D, Fujimoto A, Koft ER, Balkovec JR, Tata JR, Duke GR (2001) The first stereoselective total synthesis of quinine. J Am Chem Soc 123:3239–3242
Smith P (1969) Arrows of mercy. Doubleday, New York
Sneader W (2005) Drug discovery – a history. Wiley, New York, pp 99–100
Bernard C (1850) Action du curare et de la nicotine sur le système nerveux et sur le système musculaire. Compt Rend Séances Soc Biol (Paris) 2:195
Dale HH, Feldberg W, Vogt M (1936) Release of acetylcholine at voluntary nerve endings. J Physiol 86:353–380
McIntyre AR (1947) Curare. Its history, nature and clinical use. The University of Chicago Press, Chicago
Wintersteiner O, Dutcher JD (1943) Curare alkaloids from Chondodendron tomentosum. Science 97:467–470
King H (1935) Curare alkaloids. I. Tubocurarine. J Chem Soc 1381–1389
Weatherall M (1996) Drug treatment and the rise of pharmacology. In: Roy Porter R (ed) The Cambridge illustrated history of medicine. Cambridge University Press, Cambridge, p 256
Brown JH, Taylor P (2001) Muscarinic receptor antagonists. In: Hardman JG, Limbird LE, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, p 164
Venturella VS (1985) Natural products. In: Gennaro AR (ed) Remingtons pharmaceutical sciences, 17th edn. Mack Publishing Company, Plainview, p 418
Eliel EL, Wilen SH (1994) Stereochemistry of organic compounds. Wiley-Interscience, New York, pp 2–4
Haüy R-J (1801) Traité de Minéralogie. Conseil des Mines, Paris
Findlay A (1948) A hundred years of chemistry, 3rd edn. Gerald Duckworth & Co Ltd, London, p 57
Mauskopf SH (1976) Crystals and compounds: molecular structure and composition in nineteenth-century French science. Trans Am Phil Soc 66:65
Pasteur L (1848) Mémoire sur la relation qui peut exister entre la forme crystalline et la composition chimique, et sur la cause de la polarisation rotatoire. C R Séances Acad Sci 26:535–538
Pasteur L (1850) Recherches sur les propriétés spécifiques des deux acides qui composent l’acide racémique. Ann Chim Phys, 3e sér, 28:56–99
Delépine M (1941) Sur l’histoire de l’acide racémique et du mot racémique. Bull Soc Chim France 8:463–475
Biot J-B (1838) Sur l’emploie de la lumière polarisée pour manifester les differences des combinaisons isomérique. Ann Chim Phys 69:27
Pasteur L (1853) Notice sur l’origine de l’acide racémique. C R Séances Acad Sci 36:19–26
Gal J (2008) When did Louis Pasteur present his memoir on the discovery of molecular chirality to the Académie des Science? Analysis of a discrepancy. Chirality 20:1072–1084
Pasteur L (1922) Recherches sur la dissymétrie moléculaire des produits organiques naturels. In: Pasteur Vallery-Radot L (ed) Œuvres de Pasteur, vol 1. Masson et Cie; Paris, pp 314–344
Pasteur L (1853) Nouvelles recherches sur les relations qui peuvent exister entre la forme crystalline, la composition chimique et le phénomène rotatoire moléculaire. Ann Chim Phys 38:437–483
Herschel JFW (1822) On the rotation impressed by plates of rock crystal on the planes of polarization of the rays of light, as connected with certain peculiarities of its crystallization. Trans Camb Phil Soc 1:45–100
Evans DS (1972) Herschel, John Frederick William. In: Gillispie CC (ed) Dictionary of scientific biography, vol VI. Charles Scribner’s Sons, New York, pp 323–328
Schurig V (1997) On the origins of stereochemistry: the Herschel family – musicians, astronomers and natural scientists. Enantiomer 2:135–142
Herschel JFW (1827) Light. London, p 550 (taken from Encyclopedia Metropolitana, vol 4, 1849)
Chautard J (1853) Mémoire sur l’acide camphorique gauche et sur le camphre gaurche. Compt rend Acad Sci 37:166–167
Van’t Hoff JH (1874) Sur le formules de structure dans l'espace. Arch Neerl 9:1–10
Le Bel JA (1874) Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bul Soc Chim Paris 22:337–347
Gal J (2008) The discovery of biological enantioselectivity: Louis Pasteur and the fermentation of tartaric acid – a review and analysis 150 years later. Chirality 20:5–19
Pasteur L (1857) Mémoire sur la fermentation alcoolique. C R Séances Acad Sci 45:1032–1036
Pasteur L (1858) Mémoire sur la fermentation de l'acide tartrique. C R Séances Acad Sci 46:615–618
Gal J (2007) Carl Friedrich Naumann and the introduction of enantio terminology: a review and analysis on the 150th anniversary. Chirality 19:89–98
Duclaux E (1896) Pasteur ─ Histoire d'un esprit. Masson et Cie; Paris, p 63
Pasteur L (1922) Note relative au Penicillium glaucum et la dissymétrie moléculaire des produits organiques naturels. In: Pasteur Vallery-Radot L (ed) Œuvres de Pasteur, vol 2. Masson et Cie, Paris, pp 129–130
Morley HF, Muir PMM (1892) Watt’s dictionary of chemistry, vol 3. Longmans, Green, and Co, London, p 235
Pasteur L (1922) Transformation del l'acide tartrique en acide racémique. Découverte de l'acide tartrique inactif. Nouvelle méthode de séparation de l'acide racémique en acides tartriques droit et gauche. In: Pasteur Vallery-Radot L (ed) Œuvres de Pasteur, vol 1. Masson et Cie; Paris, pp 258–262
Piutti A (1886) Ein neues Asparagin. Ber deutsch chem Ges 19:1691–1695
Gal J (2012) The discovery of stereoselectivity at biological receptors: Arnaldo Piutti and the taste of the asparagine enantiomers – history and analysis on the 125th anniversary. Chirality. doi:10.1002/chir
Prüll C-R, Maehle A-H, Halliwell RF (2009) A short history of the drug receptor concept. Palgrave MacMillan, Basingstoke, p 1
Ehrlich P, Morgenroth J (1900) Ueber Haemolysine. Dritte Mittheilung. Berliner klin Wochenschrift 37:453–458
Langley JN (1905) On the reaction of cells and nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curare. J Physiol 33:374–413
Vickery H, Schmidt CLA (1931) The history of the discovery of the amino acids. Chem Rev 9:169–318
Vauquelin LN, Robiquet PJ (1806) La découverte d'un nouveau principe végétal dans le suc des asperges. Ann Chim 57:88–93
Piutti A (1888) Sintesi e costituzione delle asparagine. Gazz Chim Ital 18:457–472
Pringsheim H (1910) Notiz über das Vorkommen von Rechts-Asparagin in der Natur. Z Physiol Chem 65:89–95
Piutti A (1923) The simultaneous existence of optically active asparagines in germinated lupines. Atti congresso naz chim pura applicata 1923:384–386
Greenstein JP, Winitz M (1961) The chemistry of the amino acids, vol 3. Wiley, New York, p 1860
Fajans K (1910) Über die stereochemische Spezifizität der Katalysatoren (Optische Aktivierung durch asymmetrische Katalyse). Z physik Chem 73:25–96
Hirsch P (1918) Die Einwirkung von Mikroorganismen auf die Eiweiβkörper. Gebrüder Borntraeger, Berlin, pp 68–77
Cushny AR (1926) Biological relations of optically isomeric substances. The Williams and Wilkins Company, Baltimore, pp 18–37
Brockmann H (1933) Das biologische Verhalten stereoisomerer Verbindungen. In: Freudenberg K (ed) Stereochemie: eine Zusammenfassung der Ergebnisse, Grundlagen, und Probleme. Franz Deuticke, Leipzig, pp 921–961
Fischer E (1898) Bedeuting der Stereochemie für die Physiologie. Hoppe-Seyler’s Z Physiol Chem 26:60–87
Dakin H (1904) The hydrolysis of optically inactive esters by means of enzymes. I. The action of lipase on esters of mandelic acid. The resolution of inactive mandelic acid. J Physiol 30:253–263
Brockmann H (1933) Das biologische Verhalten stereoisomerer Verbindungen. In: Freudenberg K (ed) Stereochemie: eine Zusammenfassung der Ergebnisse, Grundlagen, und Probleme. Franz Deuticke, Leipzig, pp 928–930
Farber E (1972) Fischer, Emil Hermann. Dictionary of scientific biography, vol 5. Charles Scribner’s Sons, New York, pp 1–5
Lichtenthaler FW (1992) Emil Fischer’s proof of the configuration of sugars. A centennial tribute. Angew Chem Int Ed 31:1541–1546
Kagan H, Gopalaiah K (2011) Early history of asymmetric synthesis: who are the scientists who set up the basic principles and the first experiments? New J Chem 35:1933–1937
Fischer E (1894) Einfluss der Konfiguration auf der Wirkung der Enzyme. Ber dtsch chem Ges 27:2985–2993
Fischer E (1891) Ueber die Configuration des Traubezuckers und seiner Isomeren. Ber dtsch chem Ges 24:1836–1845
Ramberg PJ (2003) Chemical structure, spatial arrangement. The early history of stereochemistry, 1874–1914. Ashgate Publishing Company, Burlington, p 275
http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1902/press.html
Cushny AR (1926) Biological relations of optically isomeric substances. The Williams and Wilkins Company, Baltimore, pp 29–37
Hudson CS (1948) Historical aspects of Fischer’s fundamental conventions for writing stereoformulas in a plane. Adv Carbohydr Chem 3:1–22
Cushny AR (1926) Biological relations of optically isomeric substances. The Williams and Wilkins Company, Baltimore, p 29
Menozzi A, Appiani G (1894) Derivatives of glutamic acid. Pyroglutamic acids and pyroglutamides. Gazz Chim Ital 24:370–391
Cushny AR (1920) On optical isomers. V. The tropeines. J Pharmacol 15:105–127
Cushny AR (1909) Further note on adrenalin isomers. J Physiol 38:259–262
Cushny AR (1926) Biological relations of optically isomeric substances. The Williams and Wilkins Company, Baltimore
Clement EM, Grahame-Smith D, Elliott JM (1998) Investigation of the presynaptic effects of quinine and quinidine on the release and uptake of monoamines in rat brain tissue. Neuropharmacology 37:945–951
Pasteur L (1853) Recherches sur les alcaloïdes des quinquinas. C R Séances Acad Sci 37:110–114
Karle JM, Karle IL, Gerena L, Milhous WK (1992) Stereochemical evaluation of the relative activities of the cinchona alkaloids against Plasmodium falciparum. Antimicrob Agents Chemother 36:1538–1544
Caner H, Biedermann PU, Agranat I (2003) Conformational spaces of Cinchona alkaloids. Chirality 15:637–645
Levy S, Azoulay S (1994) Stories about the origin of quinquina and quinidine. J Cardiovasc Electrophysiol 5:635–636
Deschamps PN (1922) La médication quinique et quinidique du coeur: Étude de l'action physiologique de la quinine et de la quinidine sur l'appareil circulatoire, et de leur emploi thérapeutique au cours des arythmies, et particulièrement au cours de l'arythmie complète. A Maloine, Paris
Zarbl E, Laemmerhofer M, Woschek A, Hammerschmidt F, Parenti C, Cannazza G, Lindner W (2005) Strong versus weak chiral cation exchangers: comparative evaluation for enantiomer separation of chiral bases by non-aqueous CEC. J Sep Sci 25:1269–1283
Hedman A, Meijer DKF (1998) The stereoisomers quinine and quinidine exhibit a marked stereoselectivity in the inhibition of hepatobiliary transport of cardiac glycosides. J Hepatol 28:240–249
Muralidharan G, Hawes E, Mckay G, Korchinski E, Midha K (1991) Quinidine but not quinine inhibits in man the oxidative metabolic routes of methoxyphenamine which involve debrisoquine 4-hydroxylase. Eur J Clin Pharmacol 41:471–474
Hsueh C, Marvel CS (1928) Optically active hypnotics. J Am Chem Soc 50:855–859
Maher TJ (2008) General anesthetics. In: Lemke TL, Williams DA, Roche VF, Zito SW (eds) Foye’s principles of medicinal chemistry, 6th edn. Lippincott Williams & Wilkins, Baltimore, pp 493–494
Christensen HD, Lee IS (1973) Anesthetic potency and acute toxicity of optically active disubstituted barbituric acids. Toxicol Appl Pharmacol 26:495–503
Haley TJ, Gidley JT (1976) Pharmacological comparison of R(+), S(−) and racemic thiopentone in mice. Eur J Pharmacol 36:211–214
Yamakura T, Bertaccini E, Trudell JR, Harris RA (2001) Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol 41:23–51
Andrews PR, Mark LC (1982) Structural specificity for barbiturates and related drugs. Anesthesiology 57:314–320
Pfeiffer CC (1956) Optical isomerism and pharmacological action, a generalization. Science 124:29–31
Ariëns EJ (1983) Stereoselectivity of bioactive agents: general aspects. In: Ariëns EJ, Soudijn W, Timmermans PBMWM (eds) Stereochemistry and biological activity of drugs. Blackwell Scientific Publications, Oxford, pp 27–31
Parascandola J (1981) The theoretical basis of Paul Ehrlich’s chemotherapy. J Hist Med 36:19–43
Lillie FR (1914) Studies of fertilization. VI. The mechanism of fertilizations in arbacia. J Exp Zool 16:523–590
Fruton JS (1999) Proteins, enzymes, genes – the interplay of chemistry and biology. Yale University Press, New Haven, p 152
Easson L, Stedman E (1933) Studies on the relationship between chemical constitution and physiological action. V. Molecular dissymmetry and physiological activity. Biochem J 27:1257–1266
Davankov V (1989) Introduction to chromatographic resolution of enantiomers. In: Krstulovic AM (ed) Chiral separations by HPLC. Ellis Horwood, Chichester, pp 183–186
Bergmann M, Zervas L, Fruton JS, Schneider F, Schleich H (1935) On proteolytic enzymes V. On the specificity of dipeptidase. J Biol Chem 109:325–346
Ogston AG (1948) Interpretation of experiments on metabolic processes, using isotopic tracer elements. Nature 162:963
Eliel EL, Wilen SH (1994) Stereochemistry of organic compounds. Wiley-Interscience, New York, p 1198
Ruffolo RR Jr (1983) Stereoselectivity in adrenergic agonists and adrenergic blocking agents. In: Ariëns EJ, Soudijn W, Timmermans PBMWM (eds) Stereochemistry and biological activity of drugs. Blackwell Scientific Publications, Oxford, pp 104–115
Mesecar AD, Koshland DE (2000) Structural biology – a new model for protein stereospecificity. Nature 403:614–615
Bentley R (1983) Three-point attachment: past, present, but no future. Trans N Y Acad Sci II 41:5–24
Topiol S (1989) A general criterion for molecular recognition: implication for chiral interactions. Chirality 1:69–79
Anonymous (1992) Fed Reg 5(102):22249
Caldwell J, Hutt AJ, Fournel-Gigleux S (1988) The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences. Biochem Pharmacol 37:105–114
Yun EM, Meadows W, Santos AC (1998) New amide local anaesthetics for obstetric use. Baillieres Clin Obstet Gynaecol 12:461–471
Shah RR, Midgley JM, Branch SK (1998) Stereochemical origin of some clinically significant drug safety concerns: lessons for future drug development. Adverse Drug React Toxicol Rev 17:145–190
Acknowledgments
Helpful information from Professors Andrew J. Hutt (University of Hertfordshire, UK), Michael Lämmerhofer (University of Tübingen, Germany), and Wolfgang Lindner (University of Vienna, Austria) is gratefully acknowledged. The authors are deeply indebted to Dr. Claudia and Pietro Piutti, great-granddaughter and grandson of Arnaldo Piutti, respectively, and to Pietro Piutti’s spouse Caterina (née Rovetto), for permission to reproduce Arnaldo Piutti’s photograph. In addition, permissions from Wiley and Taylor & Francis to reproduce text extracts from previous publications [8, 9, 14, 19, 76, 85] are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gal, J., Cintas, P. (2012). Early History of the Recognition of Molecular Biochirality. In: Cintas, P. (eds) Biochirality. Topics in Current Chemistry, vol 333. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2012_406
Download citation
DOI: https://doi.org/10.1007/128_2012_406
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37625-2
Online ISBN: 978-3-642-37626-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)