Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder and one of the substantial socioeconomic and medical calamities of our time. It is characterized by progressive neurodegenerative disorder featuring dementia and cognitive impairment. It is caused by synaptic failure and the excessive aggregation of two types of misfolded proteins, namely, amyloid-β (Aβ) and tau protein. AD is a complex disease that affects neurons in different parts of the brain. The treatment of AD is difficult with currently available medications and treatment approaches due to inadequate knowledge of its etiology and versatile nature of its pathology. Stem cell holds a promising approach to regenerate the tissue systems and has been explored in various studies of neurodegenerative disorders and provides great research opportunity. Stem cell research-based therapies emit a new hope for AD treatment as a regenerative approach. This chapter focuses on recent advances in stem cell therapies according to cell types and pathophysiology of AD along with human clinical trials of stem cell therapies for the AD.
Similar content being viewed by others
Keywords
9.1 Introduction
In 1907, Alois Alzheimer first reported Alzheimer’s disease (AD) (Choi et al. 2014a, b). However, 60–80% of dementia cases are caused by AD, characterized by memory loss and cognitive disorder which affects the quality of life. AD is a progressive neurodegenerative disorder, where the symptom of dementia is gradually elevated over a number of years. In the early stages of the AD, mild memory loss occurs, but in the later stage, the patients lose the ability to converse and the ability to respond to their environment. AD severely affects the human health, as it is the sixth leading cause of mortality in the United States. The average life duration of Alzheimer’s patient is 8 years after the AD’s symptoms become noticeable, but survival range can vary from 4 to 20 years, depending on the age, lifestyle, diet, and health condition. In 2010, the estimated economic burden of AD’s treatment was $172 billion in the United States and $604 billion worldwide that will be tripled by 2050 (Wimo et al. 2010). In India, approximately 3.7 million people were suffering from AD, and this number is expected to double by the year 2030 (Alzheimer’s and Related Disorders Society of India (ARDSI) 2010).
Two types of AD are reported: (i) familial and (ii) sporadic. Familial AD is caused by an autosomal genetic mutation in the genes responsible for Aβ plaques. This genetic mutation is related to the amyloid precursor protein (APP), presenilin-1 (PSEN-1), and presenilin-2 (PSEN-2). However, familial AD is rare in prevalence and less than 5% of familial AD cases are reported (Selkoe 2001; Prasher et al. 1998; Rosen et al. 2010; Marchetti and Marie 2011; Genin et al. 2011). Sporadic AD is ubiquitous in nature and caused by the interaction between genetic profile and environmental factors (Duncan and Valenzuela 2017; Persson et al. 2014). The cardinal pathologic features of AD include the aggregation of two types of misfolded proteins (amyloid beta and tau) (Allen et al. 2011; Eckman and Eckman 2007). Amyloid beta (Aβ) protein is a pathological cleavage product of the APP. Aβ protein accumulates into plaques and minor oligomers. Mutations in APP genes or in APP processing pathway genes are linked to the inherited familial AD (Huang and Mucke 2012). Tau is a microtubule-associated protein that accumulates intracellularly as neurofibrillary tangles (NFTs) which is a pathological feature closely linked with cognitive decline in the AD. However, mutations in tau protein lead to cause frontotemporal dementia, not AD (Huang and Mucke 2012).
A rising accord inside the field is that treatment of AD patients with currently available medicines comes late, which is the result of vital neuronal cell loss within the brain. To combat these problems, human embryonic stem cell (hESC)/induced pluripotent stem cell (iPSC)/mesenchymal stem cell (MSC)-derived neural cells have been suggested as powerful replacement therapy for AD (Fig. 9.1). In this chapter, the current state of research in the etiology of AD, probable challenges, and techniques for using stem cell-based treatment will be discussed briefly. Recent studies that have developed promising cell types and clinical investigations that could be used to combat this detrimental disease in the future will also be highlighted.
9.2 Pathophysiology of Alzheimer’s Disease
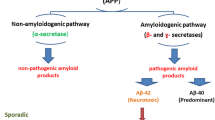
AD is distinguished by extracellular amyloid plaques and intracellular NFT features. Amyloid-β (Aβ) protein is the major constituent of plaques associated with AD (Fig. 9.2). The pathophysiology of AD involves several neurotransmitters system and processes (Lin et al. 2001). Three hallmarks of the AD are β-amyloid plaques, neurofibrillary tangles, and neuronal cell death.
Recently, recognized characteristics of AD include degeneration of synapses, aneuploidy, neuronal loss, granulovacuolar degeneration, and amyloid plaques. Three types of amyloid plaques are known in the brain of AD patients:
-
1.
Diffuse plaques: contain no amyloid core
-
2.
Neuritic plaques: consist of a central amyloid core surrounded by neurites
-
3.
Burnt-out plaques: consist of an isolated amyloid core
Apart from the amyloid plaques and tangles, globular and non-fibrillar proteins are continuously released in the AD patient’s brain. Cellular changes include short-term and rapid degeneration of neurons which leads to neuronal death when Aβ proteins remain globular.
A few theories related to AD such as the cholinergic, Aβ, tau, and inflammation hypothesis have been explained. Some of them are listed below to understand the mechanism of this disorder:
-
1.
Changes in brain structure: The characteristic of the AD on a macro level is the progressive loss of brain tissue. The cortex atrophies are responsible for memory formation in the brain.
-
2.
Degenerative processes in AD: AD is characterized on a micro level by three neuropathologic hallmarks: extracellular β-amyloid plaques, intracellular NFTs, and neuronal degeneration. β-Amyloid plaques play an important role in AD pathogenesis which is known as “amyloid cascade” (Swerdlow 2007).
9.3 β-Amyloid Hypothesis
β-Amyloid plaques are aggregates of insoluble peptides formed after the cleavage of APP. Three enzymes, namely γ-secretase, β-secretase, and α-secretase, participate in the APP cleavage. However, APP cleavage by β-secretase followed by γ-secretase produces a soluble 40-amino acid peptide. In addition, γ-secretase cleaves APP that forms nonsoluble 42-amino acid peptide Aβ42 or Aβ which aggregates as β-amyloid plaques. There are three genes involved in the formation of Aβ: APP, PS1, and PS2. PS1 and PS2 genes code for presenilin which is a subunit of γ-secretase. Tau protein hyperphosphorylation occurs after plaque formation in the brain (Selkoe 2002). Neurofibrillary tangles (NFTs) result from damage of neuronal microtubules caused by tau protein modification (Imbimbo et al. 2005). Tau protein disrupts the collapse structure of microtubules and destroys the neuron’s transport and communication system. Modifications in tau lead to its oligomerization and NFT production (Maccioni et al. 2010).
9.4 Cholinergic Hypothesis
Loss of cholinergic neurons is one of the pathologies of AD. In that case, more than 75% of cholinergic neurons are reduced in AD patient’s brain (Perry et al. 1978). However, acetylcholine is involved in memory; thus, loss of cholinergic activity relates with impairment of memory. Acetylcholine attaches to the post-synaptic receptors: muscarinic and nicotinic. Pre-synaptic nicotinic receptors influence the release of acetylcholine, serotonin, norepinephrine, and glutamate which have a role in AD pathophysiology.
9.5 Glutamatergic Hypothesis
Glutamatergic neurons form the projections which influence the cognition in the brain. AD pathology is linked to only one type of receptor, that is, NMDA receptor which then undergoes low-level activation in AD patient’s brain. However, dysregulation of the glutamate NMDA receptor is responsible for neuronal damage which interferes with normal signal transduction (Danysz et al. 2000). It can lead to the production of APP which is related to plaque development and tau hyperphosphorylation.
9.6 Oxidative Stress Hypothesis
Aβ generates the reactive oxygen and nitrogen species which have an unpaired extra electron and also induces lipid peroxidation. The free radicals cause cellular and molecular damage in neuronal cells. The brain can be damaged from oxidative stress because of high oxygen utilization rate and antioxidant enzymes as compared with the other organs. Upregulation of cytokines and DNA damage in neurons have an essential role in AD progression.
9.7 Chronic Inflammation Hypothesis
β-Amyloid deposition in neurons and NFTs causes inflammation in response to cellular damage. Inflammation leads to the increased number of prostaglandins, produced by COX-1 and COX-2, localized in distinct areas of the brain. Inflammation occurs within or adjacent to the neuritic plaque. Antichymotrypsin, macroglobulin in neuritic plaques, and activated microglia codes for interleukin-1 and interleukin-6 also are detectable in case of the inflammation-related AD.
9.8 Cholesterol and Other Factors
Cholesterol is also implicated in AD pathogenesis. Elevated cholesterol levels raise Aβ production, and thus, the risk of AD progression increases (Reiss 2005). During the AD progression, brain regions become altered, and reduced serotonin levels play an important role in depression and anxiety which are common in an AD patient (Mössner et al. 2000; Lai et al. 2005).
9.9 Stem Cells Used in Alzheimer’s Treatment
Stem cells are undifferentiated cells that possess self-renewal and differentiation property. Self-renewal is described as the ability to undergo numerous cell cycle divisions, resulting in identical daughter cells, and differentiation capability is the development of specialized cells from the undifferentiated stem cells (Tabassum et al. 2017). On the virtue of origin, stem cells can be categorized into embryonic stem cells (ESCs) and adult stem cells, and based on potency, these cells are categorized into totipotent, multipotent, pluripotent, and unipotent. Due to the differentiation properties of stem cells into neuronal-like cells, they can be used for the treatment of Alzheimer’s disease. The human body generates four types of stem cells: neural stem cells (NSCs), MSCs, ESCs, and iPSCs. These cells have unique properties; thus, they are the most suitable candidates for stem cell therapy.
Embryonic Stem Cells (ESCs)
ESCs are pluripotent stem cells which are obtained from the inner cell mass of the blastocyst that gives rise to all cell types except placenta. Researchers successfully differentiated the ESCs into several specific neural cell types including dopaminergic neurons in vitro (Krencik et al. 2011; Malmersjo et al. 2009). The direct transplantation of ESCs showed high risks of teratoma formation due to their potent differentiation ability (Kooreman and Wu 2010). Moreover, various rodent studies demonstrated that the transplantation of ESC-derived NSCs shows no tumorigenesis, but to confirm these results, further research is needed (Araki et al. 2013; Tang et al. 2008). Along with tumorigenesis, rejection of transplanted ESC-derived tissues by the immune system occurred (Pearl et al. 2012).
Induced Pluripotent Stem Cells (iPSCs)
iPSCs are pluripotent stem cells which are reprogrammed from adult fibroblasts by using four transcription factors including Oct3/4, Sox2, Klf4, and c-Myc that are pretty much similar to the ESCs (Takahashi and Yamanaka 2006). These cells are reprogrammed into pluripotency state, having the capability to differentiate into different types of cells including neurons (Cooper et al. 2010) and neurospheres (Nori et al. 2011). Researchers used the iPSC-derived glia cells regarding the inflammatory response in Alzheimer’s disease (Holtman et al. 2015). In 2014, Takamatsu revealed that iPSC-derived macrophages express neprilysin and β-amyloid-degrading protease (Takamatsu et al. 2014). However, certain unsolved problems are still present regarding the clinical usage of iPSCs such as tumor formation, immunogenicity, long-time safety, genetic defects, and optimal reprogramming (Tolosa et al. 2016; Araki et al. 2013). Therefore, iPSC-based treatment for AD has been more focused on the establishment of cell-based disease models as compared to treatments (Choi et al. 2014a, b; Yagi et al. 2012; Sproul et al. 2014). Israel and coworkers highlighted the cholinergic neurons of the basal forebrain because of their dysfunction in the early stage of AD (Israel et al. 2012). We know that there is a widespread degeneration in the later stage of the AD, so the protocol using iPSCs should be more elaborated (Pen and Jensen 2017).
Neuronal Stem Cells (NSCs)
NSCs are found within the brain. In the past few decades, it was thought that the process of neurogenesis takes place in the fetus; however, the recent studies demonstrated that neurogenesis also occurs in an adult’s brain. NSCs were found in the sub-granular zone and sub-ventricular zone of the brain (Taupin 2006; Mu and Gage 2011). These cells are differentiated into neurons, astrocytes, and oligodendrocytes (Taupin 2006). Due to the differentiation capability, NSCs are considered as the best choice for the replacement of injured neurons. In 2001, for the first time, Qu and coworkers proved the replacement of injured neuron by implanting human NSCs into the mature rat’s brain (Qu et al. 2001). The results showed that NSCs survived and differentiated into neurons and astrocytes in rat’s brain. Moreover, memory impairment was also observed in mature rats after the transplantation when evaluated with the control (Qu et al. 2001). However, NSC isolation from the adult’s brain is complicated, so current studies mainly use fetal NSCs, which could also raise ethical problems. To combat these problems, researchers focused on the MSCs, and it was found that bone marrow MSCs (BM-MSCs), adipose tissue (AT-MSCs), and umbilical cord blood MSCs (UC-MSCs) could be trans-differentiated into neuronal cells (Brazelton et al. 2000; Mezey et al. 2000; Kim et al. 2012a, b).
Mesenchymal Stem Cells (MSCs)
MSC-based therapy has an advantage over other cell-based therapy because it can be given intravenously and has blood barrier penetration and low tumorigenicity (Oh et al. 2015; Ra et al. 2011). The in vitro transplantation of MSCs in AD cell model augmented the metabolic activity and survival which help to rescue the patients with AD. Co-culturing of human MSCs and mouse microglia cells increased the expression of neprilysin (Aβ-degrading enzyme) (Kim et al. 2012a, b). BM-MSCs show the immunomodulatory capability by releasing the soluble factors including TGF-β, IL-6, IL-10, and PGE2 (Ramasamy et al. 2007; Aggarwal and Pittenger 2005). These factors inhibit the functioning of monocyte-derived dendritic cells and modify the phenotype of the natural killer cell (Sotiropoulou et al. 2006). In 2012, Chen and coworkers demonstrated that AT-MSCs can be differentiated into astrocytes and neuronal-like cells (Chen et al. 2012). The transcriptional profile of AT-MSCs showed some similarity with BM-MSCs (Peroni et al. 2008). AT-MSCs also secrete various neurotrophic factors (Gutiérrez-Fernández et al. 2013; Yang et al. 2012). UC-MSCs can be also differentiated into neuron-like cells. Researchers studied these cells in mouse model having Alzheimer’s disease and clinically (Kang et al. 2016). Table 9.1 summarizes the studies of stem cell therapy on AD-diseased animal models.
9.10 Some Clinical Trials of Stem Cell Therapies for Alzheimer’s Disease
Since 2011, animal model evidence supported the approval of MSC-based therapies in clinical trials for patients with Alzheimer’s disease. UC-MSCs were preferred, and the route of administration of stem cell is intravenous (Table 9.2).
9.11 Conclusion and Future Prospects
Stem cell therapy exhibits therapeutic benefits in several neurodegenerative disorders. Stem cell transplantation increases the expression of synaptic protein markers in AD animal models. The transplantation of MSCs elevated the level of Aβ-degrading enzyme and reduced the level of Aβ due to microglial expression. With the ongoing development of reprogramming technology, there is an immense potential in the utilization of iPSCs in the treatment of AD. For reprogramming, somatic cells from patients could be used to generate iPSCs. After that, it can be differentiated into neural precursor cells for transplantation. This means that tissue rejections will never again an issue and there will be negligible ethical problems. Also, it can ameliorate the modeling of neurodegenerative diseases like AD, because iPSCs could differentiate into neurons, having the inimitable genetic phenotype of the patient. Thus, stem cell-derived neuronal cells create a cellular model which offers the closest relation to the sporadic form of the AD disease and expectantly translated into human studies to find a cure for the AD.
References
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. https://doi.org/10.1182/blood-2004-04-1559
Allen S, Watson J, Dawbarn D et al (2011) The neurotrophins and their role in Alzheimer’s disease. Curr Neuropharmacol 9(4):559–573. https://doi.org/10.2174/157015911798376190
Araki R, Uda M, Hoki Y et al (2013) Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494(7435):100. https://doi.org/10.1371/journal.pone.0069617
ARSI. Alzheimer’s and Related Disorders Society of India (ARDSI) (2010)
Babaei P, Soltani Tehrani B, Alizadeh A (2012) Transplanted bone marrow mesenchymal stem cells improve memory in rat models of Alzheimer’s disease. Stem Cells Int 2012:369417. https://doi.org/10.1155/2012/369417
Blurton-Jones M, Kitazawa M, Martinez-Coria H et al (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci 106(32):13594–13599. https://doi.org/10.1073/pnas.0901402106
Brazelton TR, Rossi FM, Keshet GI et al (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290(5497):1775–1779. https://doi.org/10.1126/science.290.5497.1775
Chen J, Tang YX, Liu YM et al (2012) Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther 18(10):847–854. https://doi.org/10.1111/j.1755-5949.2012.00382.x
Choi SH, Kim YH, Hebisch M et al (2014a) A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515(7526):274
Choi SS, Lee SR, Kim SU et al (2014b) Alzheimer’s disease and stem cell therapy. Exp Neurobiol 23(1):45–52. https://doi.org/10.5607/en.2014.23.1.45
Cooper O, Hargus G, Deleidi M et al (2010) Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci 45(3):258–266. https://doi.org/10.1016/j.mcn.2010.06.017
Danysz W, Parsons CG, MÖbius HJ et al (2000) Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease – a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res 2(2–3):85–97
Duncan T, Valenzuela M (2017) Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther 8(1):111. https://doi.org/10.1186/s13287-017-0567-5
Eckman CB, Eckman EA (2007) An update on the amyloid hypothesis. Neurol Clin 25(3):669–682. https://doi.org/10.1016/j.ncl.2007.03.007
Esmaeilzade B, Nobakht M, Joghataei MT et al (2012) Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer’s disease rat model. Iran Biomed J 16(1):1. https://doi.org/10.6091/IBJ.1029.2012
Genin E, Hannequin D, Wallon D et al (2011) APOE and Alzheimer’s disease: a major gene with semi-dominant inheritance. Mol Psychiatry 16(9):903. https://doi.org/10.1038/mp.2011.52
Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J et al (2013) Effects of intravenous administration of allogenic bone marrow-and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 4(1):11. https://doi.org/10.1186/scrt159
Holtman IR, Raj DD, Miller JA et al (2015) Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol Commun 3(1):31. https://doi.org/10.1186/s40478-015-0203-5
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6):1204–1222. https://doi.org/10.1016/j.cell.2012.02.040
Imbimbo BP, Lombard J, Pomara N et al (2005) Pathophysiology of Alzheimer’s disease. Neuroimag Clin 15(4):727–753. https://doi.org/10.1016/j.nic.2005.09.009
Israel MA, Yuan SH, Bardy C et al (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482(7384):216
Kang JM, Yeon BK, Cho SJ et al (2016) Stem cell therapy for Alzheimer’s disease: A review of recent clinical trials. J Alzheimers Dis 54(3):879–889. https://doi.org/10.3233/JAD-160406
Kern DS, Maclean KN, Jiang H et al (2011) Neural stem cells reduce hippocampal tau and reelin accumulation in aged Ts65Dn Down syndrome mice. Cell Transplant 20(3):371–379
Kim JY, Kim DH, Kim JH et al (2012a) Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ 19(4):680. https://doi.org/10.1038/cdd.2011.140
Kim S, Chang KA, Park HG et al (2012b) The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One 7(9):e45757
Kooreman NG, Wu JC (2010) Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface 7(Suppl 6):S753–S763. https://doi.org/10.1098/rsif.2010.0353.focus
Krencik R, Weick JP, Liu Y et al (2011) Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 29(6):528. https://doi.org/10.1038/nbt.1877
Lai MK, Tsang SW, Alder JT et al (2005) Loss of serotonin 5-HT 2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology 179(3):673–677. https://doi.org/10.1007/s00213-004-2077-2
Lee HJ, Lee JK, Lee H et al (2012) Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging 33(3):588–602. https://doi.org/10.1016/j.neurobiolaging.2010.03.024
Lee JK, Jin HK, Bae JS (2009) Bone marrow-derived mesenchymal stem cells reduce brain amyloid-β deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett 450(2):136–141. https://doi.org/10.1016/j.neulet.2008.11.059
Lin HAI, Bhatia R, Lal R et al (2001) Amyloid β protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB J 15(13):2433–2444. https://doi.org/10.1096/fj.01-0377com
Maccioni RB, Farías G, Morales I, Navarrete L et al (2010) The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res 41(3):226–231. https://doi.org/10.1016/j.arcmed.2010.03.007
Malmersjö S, Liste I, Dyachok O et al (2009) Ca2+ and cAMP signaling in human embryonic stem cell–derived dopamine neurons. Stem Cells Dev 19(9):1355–1364. https://doi.org/10.1089/scd.2009.0436
Marchetti C, Marie H (2011) Hippocampal synaptic plasticity in Alzheimer’s disease: what have we learned so far from transgenic models? Rev Neurosci 22(4):373–402. https://doi.org/10.1515/RNS.2011.035
Mezey E, Chandross KJ, Harta G et al (2000) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290(5497):1779–1782. https://doi.org/10.1126/science.290.5497.1779
Mössner R, Schmitt A, Syagailo Y et al (2000) The serotonin transporter in Alzheimer’s and Parkinson’s disease. In: Advances in research on neurodegeneration. Springer, Vienna, pp 345–350
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6(1):85. https://doi.org/10.1186/1750-1326-6-85
Nori S, Okada Y, Yasuda A et al (2011) Grafted human-induced pluripotent stem-cell–derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci 108(40):16825–16830. https://doi.org/10.1073/pnas.1108077108
Oh SH, Kim HN, Park HJ et al (2015) Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transplant 24(6):1097–1109
Park D, Joo SS, Kim TK et al (2012) Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. https://doi.org/10.3727/096368911X586765
Pearl JI, Kean LS, Davis MM et al (2012) Pluripotent stem cells: immune to the immune system? Sci Transl Med 4(164):164ps25. https://doi.org/10.1126/scitranslmed.3005090
Pen AE, Jensen UB (2017) Current status of treating neurodegenerative disease with induced pluripotent stem cells. Acta Neurol Scand 135(1):57–72. https://doi.org/10.1111/ane.12545
Peroni D, Scambi I, Pasini A et al (2008) Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314(3):603–615. https://doi.org/10.1016/j.yexcr.2007.10.007
Perry EK, Tomlinson BE, Blessed G et al (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 2(6150):1457–1459
Persson T, Popescu BO, Cedazo-Minguez A et al (2014) Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxidative Med Cell Longev. https://doi.org/10.1155/2014/427318
Prasher VP, Farrer MJ, Kessling AM et al (1998) Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol 43(3):380–383. https://doi.org/10.1002/ana.410430316
Qu T, Brannen CL, Kim HM et al (2001) Human neural stem cells improve cognitive function of aged brain. Neuroreport 12(6):1127–1132
Ra JC, Shin IS, Kim SH et al (2011) Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 20(8):1297–1308. https://doi.org/10.1089/scd.2010.0466
Ramasamy R, Fazekasova H, Lam EWF et al (2007) Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 83(1):71–76. https://doi.org/10.1097/01.tp.0000244572.24780.54
Reiss AB (2005) Cholesterol and apolipoprotein E in Alzheimer’s disease. Am J Alzheimers Dis Other Dement 20(2):91–96. https://doi.org/10.1177/153331750502000208
Rosen KM, Moussa CEH, Lee HK et al (2010) Parkin reverses intracellular β-amyloid accumulation and its negative effects on proteasome function. J Neurosci Res 88(1):167–178. https://doi.org/10.1002/jnr.22178
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766. https://doi.org/10.1152/physrev.2001.81.2.741
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298(5594):789–791. https://doi.org/10.1126/science.1074069
Sotiropoulou PA, Perez SA, Gritzapis AD et al (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24(1):74–85. https://doi.org/10.1634/stemcells.2004-0359
Sproul AA, Jacob S, Pre D et al (2014) Characterization and molecular profiling of PSEN1 familial Alzheimer’s disease iPSC-derived neural progenitors. PLoS One 9(1):e84547. https://doi.org/10.1371/journal.pone.0084547
Swerdlow RH (2007) Pathogenesis of Alzheimer’s disease. Clin Interv Aging 2(3):347
Tabassum N, Verma V, Yadav CB et al (2017) Tissue engineering in regenerative medicine. In: Stem cells from culture dish to clinic. Nova Science Publishers Inc, New York, pp 17–32
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Takamatsu K, Ikeda T, Haruta M et al (2014) Degradation of amyloid beta by human induced pluripotent stem cell-derived macrophages expressing Neprilysin-2. Stem Cell Res 13(3):442–453. https://doi.org/10.1016/j.scr.2014.10.001
Tang J, Xu H, Fan X et al (2008) Embryonic stem cell-derived neural precursor cells improve memory dysfunction in Aβ (1–40) injured rats. Neurosci Res 62(2):86–96. https://doi.org/10.1016/j.neures.2008.06.005
Taupin P (2006) Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev 2(3):213–219
Tolosa L, Pareja E, Gómez-Lechón MJ (2016) Clinical application of pluripotent stem cells: an alternative cell-based therapy for treating liver diseases? Transplantation 100(12):2548–2557. https://doi.org/10.1097/TP.0000000000001426
Wang Q, Matsumoto Y, Shindo T et al (2006) Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Investig 53(1–2):61–69. https://doi.org/10.2152/jmi.53.61
Wen SR, Qi HP, Ren YJ et al (2011) Expression of deltaNp73 in hippocampus of APP/PS1 transgenic mice following GFP-BMSCs transplantation. Neurol Res 33(10):1109–1114. https://doi.org/10.1179/1743132811Y.0000000051
Wimo A, Winblad B, Jönsson L (2010) The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement 6(2):98–103. https://doi.org/10.1016/j.jalz.2010.01.010
Xuan AG, Luo M, Ji WD et al (2009) Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett 450(2):167–171. https://doi.org/10.1016/j.neulet.2008.12.001
Yagi T, Kosakai A, Ito D et al (2012) Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. PLoS One 7(7):e41572. https://doi.org/10.1371/journal.pone.0041572
Yang KL, Lee JT, Pang CY et al (2012) Human adipose-derived stem cells for the treatment of intracerebral hemorrhage in rats via femoral intravenous injection. Cell Mol Biol Lett 17(3):376. https://doi.org/10.2478/s11658-012-0016-5
Acknowledgment
This work is supported by SERB (EEQ/2018/000114) grant.
Disclosure of Potential Conflicts of Interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tabassum, N., Yadav, C.B., Singh, A., Verma, V. (2019). Stem Cell Therapy: A Great Leap Forward in Alzheimer’s Treatment. In: Ashraf, G., Alexiou, A. (eds) Biological, Diagnostic and Therapeutic Advances in Alzheimer's Disease. Springer, Singapore. https://doi.org/10.1007/978-981-13-9636-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-9636-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-9635-9
Online ISBN: 978-981-13-9636-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)