Abstract
In order to understand the effect of chronic exposure to low-dose-rate radiation on female fertilities and the risk of heritable genetic effects on domestic animals after the Fukushima Daiichi Nuclear Power Plant (FNPP) accident, we assessed the developmental ability of oocytes, and examined the histological characters and structure of ovaries. We showed that potentially viable oocytes could be collected from the ovary of abandoned cattle in the ex-evacuation zone set within a 20-km radius from FNPP, resulting in production of the morphologically normal calves following in vitro culture and embryo transfer. The proliferation of cattle ovarian granulosa cells was confirmed by expression of Ki-67. Apoptosis of oocytes and granulosa cells was few determined by the TUNEL assay. In addition, porcine and inobuta oocytes had the abilities of in vitro maturation. These results suggest that chronic radiation exposure associated with the FNPP accident may have little effect, if any, on the female fertilities of domestic animals.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Female reproductive organs such as the ovary, oviduct and uterus are essential organs to produce the next generation in mammals. Oocytes, female germ cells, are contained in the ovary. Oogenesis, namely, oocyte production begins in the fetal ovary. Oogonia proliferate via mitosis ultimately become oocytes via meiosis, and are arrested in the first meiotic prophase during fetal development [9]. The ovaries at the time of birth contain a large amount of oocytes, and oogenesis in the adult does not increase the number of germ cells. In the ovary, oocytes form the ovarian follicles along with somatic cells such as granulosa cells which support the growth of oocytes. A primordial follicle, consisting of an oocyte surrounded by a single layer of follicular cells (pregranulosa cells), is the most immature stage of an ovarian follicle’s development and can remain in this state for many years. After puberty (sexual maturation), some primordial follicles resume development into the primary, secondary and antral follicles with proliferation of granulosa cells and an increase in the oocyte diameter, and finally ovulate following development to the fully mature state. Damage to oocytes and follicular cells may lead to apoptosis, mutants and chromosomal changes, resulting in infertility and hereditary diseases.
Due to the nuclear accident caused by the Great East Japan Earthquake on 11 March 2011, a large amount of radioactive substances were emitted from Fukushima Daiichi Nuclear Power Plant (FNPP) [6, 15]. Although there are great concerns about the effect of emitted radioactive substances on female germ cells and fetuses in humans, radiobiological data on those risks led by chronic radiation exposure to low-dose-rate radiation are limited. In radiation therapy, previous studies have shown that the human oocytes are sensitive to radiation and that the estimated median lethal dose (LD50) is less than 2 Gy [12]. On the other hand, Adriaens et al. [2] reviewed that congenital anomalies have been observed after exposure to high doses (1–5 Gy) in mice, but prudence is required to extrapolate these data to humans. However, it is difficult to obtain a clear answer for risks of low-dose radiation only by epidemiological studies because of the diversity of examined population and the requirement of a long-term observation. Furthermore, careful and accurate analyses are required. The domestic animals abandoned in the ex-evacuation zone set inside a 20-km radius surrounding FNPP (abandoned animals) were the invaluable research samples for studying the effect of chronic radiation to low-dose-rate exposure. In addition, it is important to preserve the samples for the advanced analysis in the future [11].
In order to elucidate the effect of chronic radiation exposure on female fertilities and the risk of heritable genetic effects in domestic animals after the FNPP accident, we assessed the developmental ability of oocytes and performed histological examination in ovaries.
2 Methods
2.1 Production of the Next Generation Derived from Abandoned Animals
The present study was approved by the Ethics Committee of Animal Experiments, Tohoku University. Cattle ovaries of Japanese Black and Holstein were collected in Kawauchi Village located 15 km southwest, Naraha Town located 17 km south, and Tomioka Town located 10 km south of FNPP between August 31, 2011 and September 12, 2012 (from 6 months to 1.5 years after the accident). The collected ovaries were washed and stored at approximately 20 °C in physiological saline containing antibiotics and transported to the laboratory within 7 h. The bovine embryos were in vitro produced and cryopreserved as described [1] with the following modifications. The bovine cumulus-oocyte complexes (COCs) were aspirated from visible ovarian follicles by a 10-ml syringe with an 18-gage needle and were put into the maturation medium consisting of TCM199 supplemented with 10% fetal bovine serum (FBS), follicle-stimulating hormone, and antibiotics. After the culture for 22–23 h, the matured COCs were co-incubated with frozen-thawed spermatozoa in the fertilization medium consisting of Brackett and Oliphant medium with 3 mg/ml bovine serum albumin (BSA) and 2.5 mM theophylline. Those spermatozoa were commercially available and derived from a non-radiocontaminated bull semen of Japanese Black cattle (Livestock Improvement Association of Japan, Tokyo, Japan). After insemination, cumulus cells were removed from presumptive zygotes by putting them in and out using a fine-bore pipette and then cultured for in vitro development to the blastocyst stage in a modified synthetic oviduct fluid medium. After examination, the blastocysts obtained at days 7 to 9 post culture were cryopreserved by a vitrification method. The blastocysts were exposed first for 7 min to solution A, which was composed of 10% (v/v) ethylene glycol, 4.5% (w/v) Ficoll-70 and 0.075 M sucrose in modified phosphate-buffered saline (PB1) [13]; then for 2 min to solution B, which was composed of 20% (v/v) ethylene glycol, 9.0% (w/v) Ficoll-70 and 0.15 M sucrose in PB1; and finally for 1 min to solution C, which was composed of 40% (v/v) ethylene glycol, 18% (w/v) Ficoll-70 and 0.3 M sucrose in PB1 at room temperature. One to two blastocysts were then transferred onto a nylon-mesh holder with a minimum amount of medium, and the holder was directly plunged into liquid nitrogen (LN2) and stored until use for embryo transfer. Exposing to solution C and plunging into LN2 were performed within 1 min. To warm the frozen blastocysts, the holder with blastocysts was soaked in PB1 containing 0.5 M sucrose at 37°C. To remove the cryoprotectants, the blastocysts recovered from the holder were exposed to PB1 with a sequential series of 0.5, 0.25 and 0.125 M sucrose for 1 min in each solution at 37°C and finally transferred into PB1 for 5 min at 37°C. Eight Japanese Black blastocysts with normal morphology were transferred nonsurgically to synchronized recipient cows (one blastocyst per animal).
We could also collect ovary samples from 12 pigs and 2 inobutas (hybrids of a wild boar and a pig) between 18 January 18 and February 28, 2012 (10–11 months from the accident). Different from cattle ovaries, their ovaries were transported to the laboratory at 37°C. In vitro maturation (IVM) was carried out for 44 h on COCs, instead of 22–23 h in cattle. The presumptive matured oocytes were vitrified by the similar manner to the above for cattle.
2.2 Morphology, Cell Proliferation and Apoptosis Assay of the Ovary
After aspiration of COCs, the ovary was fixed in 10% buffered formalin and was embedded in paraffin and sectioned. General morphology was assessed by hematoxylin and eosin staining, granulosa and theca cell proliferation index was by Ki-67 immunostaining [8], and apoptosis of granulosa cells was by terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay [3].
2.3 Measurements of Radioactivity
Radioactivity of the female reproductive organs (ovaries, oviducts, and uteri) and peripheral blood was determined by γ-ray spectrometry using three high-purity germanium detectors (ORTEC, TN, USA) described in the previous report [14]. The measurement time took 3,600–200,000 s depending on the radioactivity of the sample. The detection efficiency was determined by measuring mixed sources of cesium-134 (134Cs) and cesium-137 (137Cs). An aliquot (200 ml) of the mixing source was diluted with an appropriate amount of water, and a superabsorbent polymer was added to the mixture to make a gel standard source, which was used as a simulated sample mimicking biological samples. Several types of gel sources were prepared to cover the weight range from 0.5 to 130 g. The detection efficiency of the liquid samples such as peripheral blood was determined using aqueous solutions of 134Cs and 137Cs.
3 Results
3.1 Viability and Fertility of Oocytes in Abandoned Animals
A total of 714 morphologically viable COCs were collected from 42 adult cattle, among which some COCs had partly dispersed cumulus cells or heterogeneous ooplasm. Figure 9.1 shows the morphological appearances of excised female reproductive organs (1A), ovaries (1B), COCs collected from ovaries (1C), COCs in vitro matured for 23 h (1D), and produced embryos (1E) following in vitro fertilization (IVF) and culture (IVC) for 8 days. No remarkable abnormalities were observed in the collected organs. After culture for IVM, cumulus expansion with cell proliferation, which is known as an index of oocyte maturation, was observed in almost all COCs. As a result of IVF and IVC, the rates of cleavage and development to blastocyst stage were 54.9% and 10.5%, respectively. Finally, 41 blastocysts were yielded and then were cryopreserved. After the transfer of eight blastocysts to eight synchronized recipient cows, three calves were born (Fig. 9.1F, Table 9.1). A total of 436 porcine and 64 inobuta COCs were collected from 12 and 2 adult animals, respectively, and the COCs exhibited the cumulus expansion after IVM. The rate of presumptive maturation was 85.1% (371/436) for pigs and 100% (64/64) for inobutas. These presumptive matured oocytes were cryopreserved for further analyses.
Morphological figures of female reproductive organs and in vitro fertilization. (A) Excised female reproductive organs. (B) Some follicles are visible through the ovarian surface. (C) Cumulus-oocyte complexes (COCs) collected from the ovaries. (D) In vitro matured COCs exhibiting cumulus expansion, that is, an index of oocyte maturation. Arrows indicate the produced blastocysts. Bar, 500 μm. (E) Developed embryos following in vitro fertilization and cultured for 8 days. (F) A delivered calf following embryo transfer
3.2 Cell Proliferation and Apoptosis in Cattle Ovaries
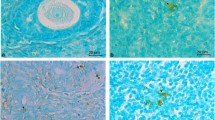
The histological examination (Fig. 9.2) revealed that none of the ovaries derived from 12 adult abandoned cattle (2A and B) and 2 infants (2C and D) exhibited structural organ damage, as shown in Fig. 9.2. Although the abandoned cattle can be recognized individually by the ear tag, the age of the cattle without the ear tag was estimated from height and body size. We classified a cattle as infant if it was younger than 6 months old. Those ovaries had primordial, primary, secondary and antral follicles, which were morphologically intact. Furthermore, Ki-67 immunoreactivity was detectable in many granulosa cells of the primary and secondary follicles (Fig. 9.3). The follicle with TUNEL-positive apoptotic cells was observed in only 1 follicle from a cattle, but the follicular cells were TUNEL-negative in a total of 97 follicles derived from 11 cattle.
Immunohistochemistry of (A, B) Ki-67, an proliferated cell marker; (C) TUNEL, an apoptotic cell marker; and (D) propidium iodide, counterstain, of ovaries derived from adult cattle. Follicles have the proliferated and non-apoptotic granulosa cells. Arrows indicate the follicles with proliferated granulosa cells
3.3 Radioactivity Concentration of 134Cs and 137Cs in Female Cattle Reproductive Organs
The reproductive organs such as ovaries, oviducts and uterus were derived from 18 adult cattle, 5 infants and 2 fetuses. Concentrations of 134Cs and 137Cs in female reproductive organs are shown in Tables 9.2 and 9.3, respectively. After decay correction to the day of major release, March 15, 2011, radioactivity concentration of 134Cs was almost equal to that of 137Cs in all organs regardless of the age. The radioactivity concentration levels of 137Cs in the ovary of adult cattle (135 ± 19 Bq/kg; range 11–256 Bq/kg) and infants (137 ± 12Bq/kg; range 124–149 Bq/kg) were higher than those levels in the ovaries of fetuses (46 ± 15 Bq/kg; range 31–61 Bq/kg). Also in oviducts and uterus, the levels in adult cattle and infants were lower than those in fetuses. On the other hand, 137Cs concentration in ovaries was the highest among the female reproductive organs examined and was approximately 2.3-fold greater than that in oviducts.
The peripheral blood samples were derived from 58 adult cattle and 8 infants. As with the reproductive organs, radioactivity concentration of 134Cs in peripheral blood was almost equal to that of 137Cs. The levels of 137C concentration in adult cattle (29 ± 3 Bq/kg; range 4–96 Bq/kg) were similar to those in infants (32 ± 6 Bq/kg; range 12–62 Bq/kg). The levels in peripheral blood were more than 3-folds smaller than those in ovaries and uterus regardless of the age.
4 Discussion
In the present study, we showed that potentially viable oocytes were obtainable from the ovaries of abandoned cattle, resulting in production of offspring. The number of collected oocytes per animal (17.0) was equal or more compared with that of those collected in a slaughter house (6–10). On the other hand, the rates of cleavage and development to blastocyst stage (54.9% and 10.5%, respectively) were lower than those in the ordinary experiments with the samples from the slaughter house (70–80% and 20–40%, respectively). In this study, the collection of organs was carried out in the unsuitable condition for oocytes: an open field with harsh temperature, euthanasia by anesthetic agents that may have an adverse effect on oocytes, and a long time between sampling and the start of IVM. Additionally, COC morphology is involved in the determination of in vitro developmental competence of immature oocyte; therefore the COCs with compact cumulus cells and homogeneous ooplasm are selected for in vitro embryo production [5]. In the present study, we performed IVM for COCs even with partly dispersed cumulus cells or heterogeneous ooplasm because the primary objective was to have offspring production. Although the blastocyst rate was low for the above reasons, the collected oocytes had the developmental competence to the blastocyst stage regardless the habitat and 137Cs activity concentration in peripheral blood of their originating animals (1–33 Bq/kg). In addition, the delivery rate following cryopreservation and transfer of produced blastocysts (37.5%, 3/8) was similar to the rate of non-radiocontaminated ones in our facility (approximately 40%), and the born calves were macroscopically normal.
Although several studies with laboratory animals on the genetic effect of ionizing radiation showed that radiosensitivity of oocytes varies widely according to the follicle/oocyte stage and the species [2], the effect on large animals such as cattle remains unknown. In the present study, histological examination of cattle ovaries showed that there was not much difference in the morphology and the average number of the follicles between ovaries collected from cattle abandoned and those collected at the slaughterhouse. The intact proliferation in cattle ovarian granulosa cells was confirmed by expression of Ki-67, and quite few apoptotic oocytes and granulosa cells were observed by the TUNEL assay. Granulosa cells produce estradiol-17β and provide various low-molecular-weight substrates (e.g., amino acids and nucleotides) to the oocytes during folliculogenesis [7, 10]. Those compounds are utilized to synthesize important macromolecules such as proteins and nucleic acids. Estradiol-17β acts as a growth hormone for tissues of the reproductive organs and appears necessary to maintain oocytes in the ovary. Apoptosis of granulosa cells is associated with induction of follicular atresia in the mammalian ovary. Moreover, the dysfunction of granulosa cells leads to the arrest of oocyte growth and follicular development, resulting in infertility. Our data in histological examination of ovaries and the fact that some cattle captured in the ex-evacuation zone were pregnant suggest that cattle ovaries appear to have the potentials of endocrine function and ovulation, and that the folliculogenesis was performed normally in abandoned cattle.
Regarding radioactive Cs concentration, the level in the female reproductive organs of fetuses was lower than those in adult cattle and infants, although the level in other organs of fetus and infants was higher than that of the corresponding maternal organ, respectively [4]. Since active angiogenesis occurs during the late follicular development after puberty and during gestation, blood vessels in the fetal reproductive organs are premature, and, therefore, the amount of transported radioactive Cs into the fetus may be limited. The levels of radioactive Cs concentration in the oviducts were lower than those in the ovary and uterus, which may be because the oviduct with luminal structure is composed of less parenchymal cells than the ovary and uterus. As well as cattle, we confirmed that porcine and inobuta ovaries maintained competence for oocyte maturation in vitro without morphologically abnormal findings.
In conclusion, no adverse radiation-induced effects were observed in the female reproductive organs derived from the domestic animals exposed to chronic radiation for 379 days in the ex-evacuation zone. The oocyte maintained the developmental competence to potentially viable blastocysts, and no hereditary abnormalities were observed in the yield offspring. In addition, we succeeded in cryopreserving the oocytes of the corresponding animals. Unfortunately, even if such accidents or problems as the FNPP accident occur in the future, we hope that our data and cryopreserved materials will be of some help.
References
Abe Y, Hara K, Matsumoto H et al (2005) Feasibility of a nylon-mesh holder for vitrification of bovine germinal vesicle oocytes in subsequent production of viable blastocysts. Biol Reprod 72(6):1416–1420
Adriaens I, Smitz J, Jacquet P (2009) The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update 15(3):359–377
Brooks K, Spencer TE (2015) Biological roles of interferon tau (IFNT) and type I IFN receptors in elongation of the ovine conceptus. Biol Reprod 92(2):47. 1–10
Fukuda T, Kino Y, Abe Y et al (2013) Distribution of artificial radionuclides in abandoned cattle in the evacuation zone of the Fukushima Daiichi nuclear power plant. PLoS One 8(1):e54312
Hazeleger NL, Hill DJ, Stubbing RB, Walton JS (1995) Relationship of morphology and follicular fluid environment of bovine oocytes to their developmental potential in vitro. Theriogenology 43(2):509–522
Kinoshita N, Sueki K, Sasa K et al (2011) Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci USA 108(49):19526–19529
McLaughlin EA, McIver SC (2009) Awakening the oocyte: controlling primordial follicle development. Reproduction 137(1):1–11
Salvetti NR, Stangaferro ML, Palomar MM et al (2010) Cell proliferation and survival mechanisms underlying the abnormal persistence of follicular cysts in bovines with cystic ovarian disease induced by ACTH. Anim Reprod Sci 122(1–2):98–110
Schillo KK (2008) Reproductive physiology of mammals: from farm to field and beyond. Delmar, New York
Shimizu (2016) Molecular and cellular mechanisms for the regulation of ovarian follicular function in cows. J Reprod Dev 62(4):323–329
Takahashi S, Inoue K, Suzuki M et al (2015) A comprehensive dose evaluation project concerning animals affected by the Fukushima Daiichi Nuclear Power Plant accident: its set-up and progress. J Radiat Res 56(Suppl 1):i36–i41
Wallace WH (2011) Oncofertility and preservation of reproductive capacity in children and young adults. Cancer 117(10 Suppl):2301–2310
Whittingham DG (1974) Embryo banks in the future of developmental genetics. Genetics 78(1):395–402
Yamashiro H, Abe Y, Fukuda T et al (2013) Effects of radioactive caesium on bull testes after the Fukushima nuclear plant accident. Sci Rep 3:2850
Zheng J, Tagami K, Watanabe Y et al (2012) Isotopic evidence of plutonium release into the environment from the Fukushima DNPP accident. Sci Rep 2:304
Acknowledgments
We would like to thank the Iwaki Livestock Hygiene Service Centre in Fukushima Prefecture, all people associated with the livestock production field in Fukushima. This study was supported by the Emergency Budget for the Reconstruction of Northeastern Japan, MEXT, Japan; Discretionary Expense of the President of Tohoku University; and Nippon Life Insurance Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this chapter
Cite this chapter
Abe, Y. et al. (2020). Analysis of Ovaries and Fertilities in Domestic Animals Affected by the Fukushima Daiichi Nuclear Power Plant Accident. In: Fukumoto, M. (eds) Low-Dose Radiation Effects on Animals and Ecosystems. Springer, Singapore. https://doi.org/10.1007/978-981-13-8218-5_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-8218-5_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8217-8
Online ISBN: 978-981-13-8218-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)