Abstract
We aimed to develop a rapid and sensitive method to analyze the radioactivity of 90Sr by combining multiple techniques, including online solid-phase extraction (SPE) and inductively coupled plasma mass spectrometry (ICP-MS). An automatic analytical system was designed to execute the proposed process from sample injection to measurement. The analysis time is approximately 20 min and the limit of detection is 0.3 Bq/L (equivalent to 0.06 pg/L) with 50 mL of the sample. Although several challenges were encountered with the ICP-MS measurements of 90Sr, several techniques were leveraged to overcome them. Online solid-phase extraction (SPE) was used to concentrate the sample automatically; the interference from polyatomic ions and isobars was removed by an oxidation, and the extraction and recovery ratio of solid phase were measured by split-flow injection with internal standard correction during the transient signal measurement. These improvements were shown to allow measurements of 90Sr in various kinds of samples to be conducted more quickly than by alternative conventional radiometric methods.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Measurements of pure-beta-emitting radioactive 90Sr require that it be isolated from other beta nuclides. The standard analysis process is milking, and low-back-gas-flow counting requires multi-step chemical separation; this process is complex, and takes a lot of time and human handling. Moreover, radioactive 90Y production is required to conduct highly sensitive 90Sr radiometric measurements and the entire analysis takes 1–2 weeks. However, there is a need for a simple and rapid analysis method, particularly for emergency situations such as the Fukushima Daiichi nuclear accident. In this chapter, we introduce a rapid method conducted in a fully automated analysis system for 90Sr analysis using inductively coupled plasma mass spectrometry (ICP-MS) and online solid-phase extraction (online SPE) with a flow-injection system (Takagai et al. 2014, 2017). This method requires only nitric acid and water as reagents. The use of automation mitigates the radioactive hazards to human health and improves the precision of the analysis. The mass spectrometer includes a system to separate the elements in aqueous samples by mass (more precisely, the ratio between an element’s mass and its electric charge (m/z)). The mass resolution depends on the type of device used; the quadrupole-type mass spectrometer used in ICP-MS has an integral resolution. Therefore, this technique suffers from poor sensitivity and interference from isobars since there are several elements with masses of 90 such as the stable isotope 90Zn, naturally occurring in the environment. Though ICP-MS can be used to analyze inorganic elements with very high sensitivity, this analysis alone cannot be used to detect low concentrations of 90Sr on the order of a few Bq/L such as the environmental 90Sr level. The half-life of 90Sr is 28.9 years and 1 Bq/L of 90Sr is equivalent to a mass concentration of almost 0.2 pg/L. The limit of detection of the stable isotope, 88Sr, by ICP-MS in an ordinary laboratory environment is approximately 500 pg/L because of contamination from the environment and the insufficient sensitivity. Our research team has previously developed a method of concentration and separation to improve the sensitivity and prevent the interference from other nuclides. In addition, as explained herein, several additional techniques are leveraged for this analysis. The proposed system measures 90Sr based on a working curve determined using a standard solution of a stable isotope of Sr based on the correlation between the detection intensity of the stable isotope of Sr and 90Sr. This feature removes the need for a radioactive standard solution, which is difficult to obtain and handle.
20.2 Materials and Methods
Figure 20.1 explains the online the SPE/ICP-MS method. This system combines several techniques. A FIAS400 flow injection device, which has an eight-direction switching valve and two peristaltic pumps, was used. Either a NexION300S or ELAN DRC II (PerkinElmer) ICP-MS instrument, which have collisions and reaction cells to remove the spectrum interference, were also used. As Fig. 20.1 shows, the system incorporates several techniques: (A) a mechanism to remove the interference from isobars with the 90Sr measurements using the collisions and reaction cells in the ICP-MS instrument. (B) PEEK columns (PerkinElmer Japan, φ4 × L50 mm) filled with Sr resin (Eichrom Technology, Particle size 50–100 μm) were used with the flow-injection system for online concentration and separation. Because the adsorption capacity of the Sr resin is limited, one or two columns may be connected alternatively based on the Sr concentration of the sample (not only 90Sr but also stable Sr). (C) To monitor the fluctuation in the ICP-MS measurement sensitivity and the measurement intensity due to the sample matrix (several interference matters) over the long term, internal standard elements are introduced at a fixed speed at a point below the columns and before the nebulizer; this is expected to minimize the error in the measurements. (D) To measure the relative recovery ratio, a novel technique based on split-flow injection was developed; this mechanism allows the sample to be split online and analyzed in parallel both before and after the column concentration step. These strategies are seamlessly integrated into a single system to enable rapid analysis.
Illustration of the online SPE/ICP-MS system with split flow. The injected sample solution is divided into two flows. One flow is measured by direct injection of the sample and the other flow is measured after preconcentration following online column separation. The measurements from both the direct injection mode and the online preconcentration mode are conducted continuously and in parallel in this automated system. In this way, the relative recovery percentage (related to the SPE efficiency) can be calculated and the targets can be quantified simultaneously. This system can rapidly and very sensitively determine the amount of 90Sr by combining (a) inference removal with the oxygen reaction, (b) column preconcentration and separation of 90Sr, (c) online correction with an internal standard, and (d) measurement of the relative recovery
20.3 Results and Discussion
20.3.1 Addressing the Interference from Isobars that Affects the ICP-MS Measurements
Sources of interference for the measurement of 90Sr include other isobars and polyatomic ions with mass numbers of 90 (including 90Zr, 90Y, and 89Y1H, and 54Fe36Ar, and 74Ge16O) or the plasma involved in ICP-MS. The multi-step column separation process can remove most of these components before they are introduced to ICP-MS. However, traces of these components remain, affecting the quantitative values obtained because the 90Sr concentration is also low. Figure 20.2 shows the mass spectrum with and without oxygen in the reaction cells. A certified radioactive reference material (DAMRI, 51.7 kBq/g, 01.04.1993) was diluted as an RI standard solution for the measurement. Figure 20.2 shows that the device converted the Zr and Y in the RI standard solution to ZrO and YO, resulting in higher mass numbers. On the other hand, only a portion of the Sr was converted to SrO. Figure 20.3 shows the relationship between the introduced oxygen gas flow causing a reaction with oxygen and the differences between the intensities of the detected ions. Sr, Zr, and Y were converted to plasma ions and exposed to collisions in the reaction cells, resulting in oxidation. The susceptibility of an ion to oxidization depends on the element. The detected ion Zr and Y intensities decreased proportionally as the oxygen gas flow increased, indicating that most of the Zr and Y were oxidized. However, the detected Sr intensity remained approximately fixed, meaning that Sr is relatively resistant to oxidization. This confirms that Sr can be physically separated from Zr and Y on the basis of the susceptibility to oxidization. Thus, combining this technique with column separation removes the interference from isobars and polyatomic ions completely such that the detection counts of m/z = 90 is approximately the same as background level in the blank sample. Thus, it is possible to measure lower concentrations over the relatively low background signal.
Mass spectra of a radioactively certified 90Sr solution containing stable Sr, Y, and, Zr without the oxygen reaction (white line) and with the oxygen reaction (solid black line) in the dynamic reaction cell (Takagai et al. 2014). In the spectrum in the absence of oxygen, the peak attributed to 90Zr overlapped with that associated with 90Sr. In the presence of oxygen, the Zr peak was shifted to a higher mass number as it existed as ZrO while some of the Sr remained as 90Sr in the position of m/z = 90. In the oxygen reaction in the dynamic reaction cell, only a small portion of the Sr was converted to SrO. The spectra showed that 25 ppt was present in the 90Sr certificated solution (equivalent to 127 Bq/g) when the sample was directly injected through a concentric nebulizer into the ICP-MS instrument without online column preconcentration
Intensities of the Sr (circles), Y (crosses), and Zr (squares) peaks as functions of the flow rate of O 2 into the dynamic reaction cell (Takagai et al. 2014). The initial 88Sr, 89Y and 90Zr concentrations were 0.05, 100, and 100 ng/L, respectively. The interference of isobars (90Zr and 89Y1H etc.) could be removed effectively by the introduction of oxygen into the dynamic reaction cell in ICP-MS
20.3.2 Addressing the Sr Selectivity of the Resin Based on Its Adsorption Characteristics
As shown in Fig. 20.4, the adsorption characteristics of the Sr resin were evaluated in terms of the recovery ratios for 53 kinds of elements. The results show that Sr was adsorbed selectively by the resin. However, Ba and Pb, whose mass numbers are 138 and 208 respectively, were also adsorbed. Therefore, for the measurement of beta rays, it is necessary to separate Sr and Pb. However, ICP-MS separates nuclides based on their masses for detection and, thus, Pb in an eluate from the columns does not affect the detection of m/z = 90.
Evaluation of adsorption capacity of the Sr resin for several metal ions. Multiple elements were adsorbed under the optimum adsorption conditions (20 vol% HNO3) and, then, eluted in ultra-pure water from the Sr resin and the recovery percentages were compared. Large amounts of Sr, Ba and Pb were recovered
20.3.3 Radioactive 90Sr Measurements Using a Standard Solution of the Stable Isotope, 88Sr
In general, ICP-MS measures analytical targets by comparing the detected intensity of a sample with that of a standard solution with a known concentration. Therefore, it is necessary to measure an 90Sr solution with a known concentration for the subsequent analysis of 90Sr. However, radioactive 90Sr has to be handled carefully in a specialized facility for radioactive materials. Thus, to allow easier and safer measurement, it is better to avoid the use of radioactive standard materials. We hypothesized that a 90Sr calibration curve of a stable isotope of Sr could be used to measure Sr because the detected intensities of 90Sr and the stable Sr isotope are correlated. However, ICP-MS exhibits a mass bias, a phenomenon where the efficiency varies for different ions depending on the mass. Several methods can be used to correct the mass bias such as the relative standardization method to derive a correction factor by measuring the certified standard materials whose isotopic ratio is guaranteed before and after the sample measurement and the internal correction method to correct the results using the measured values of two stable isotopes when an element has multiple stable isotopes. Both of the methods are used to correct the mass bias in the measured values and to calculate their true isotopic ratios.
In this study, we conducted 90Sr measurements using 88Sr as the target as the ratio of 88Sr/86Sr is the same as the natural isotope abundance ratio. This ratio can be controlled by changing the cell entrance voltage, one of the voltage parameters related to the ion vitrification in ICP-MS. Figure 20.5 shows the changes in the measured ratio of 88Sr/86Sr as the cell-entrance voltage was varied. In one condition, the ratio was close to the natural isotope abundance ratio of 8.375. In Fig. 20.6, the measured intensities of 88Sr and 90Sr at each concentration are plotted on the same graph. The abundance of 88Sr (82.58%) was converted to 100% and the detected intensity at each concentration was plotted; the resulting data demonstrated a good correlation coefficient of 0.9996. This suggests that it is possible to measure 90Sr by using a calibration curve of 88Sr. Although the measurement of the isotopic ratio using ICP-MS contains errors due to the calculation of the 90Sr values using a calibration curve for 88Sr, these errors are smaller than the measured signal errors.
Cell-entrance voltage profiles of different Sr isotope ratios obtained using the MS detector and adjustment of the cell-entrance voltages for Sr detection. The observed isotope ratio (i.e., the ratio between the intensities of 88Sr and 86Sr) as measured by QMS was gradually varied. The natural isotope ratio of 88Sr/86Sr is 8.375; a similar value was observed (8.326, −0.247% difference) when the cell-entrance voltage was −17 V. Thus, this voltage is suitable to quantify Sr because the determination error is sufficiently smaller than the measured values under these conditions. Here, MS was used as quadrupole MS (QMS)
Correlation between the intensity of stable isotope, 88Sr, and the radioactive isotope, 90Sr, at different concentrations. 90Sr (n = 3) measurements are shown as white circles (○) and 88Sr (n = 3) measurements are shown as black crosses (×). When the abundances of 88Sr and 90Sr are the same, the slopes of the calibration corresponded closely. In addition, the background of the artificial 90Sr isotope was almost zero. Therefore, the 90Sr can be indirectly derived from the measurements of the 88Sr stable isotope using the calibration curve. The detection limit of standard ICP-QMS (with a concentric nebulizer and without any accessories or attachments) was 0.055 ng/L (equivalent to 280 Bq/L)
20.3.4 Analysis of 90Sr Using Online SPE/ICP-MS
Online SPE can be used to automatically concentrate Sr in a sample in the column, elute it in an eluent, and introduce it to ICP-MS. The process from concentration to measurement is described as follows. The column was first conditioned in 20 vol% nitric acid. Then, the sample was introduced to the column. It took about 10 min to concentrate a 50 mL sample. The split-flow method was used to measure the recovery ratio at the columns during this process (explained below). Other substances present in the columns were washed out with the 20 vol% nitric acid. The concentrated Sr in the resin was eluted by switching a value position (in flow injection system). Figure 20.7 shows an eluted chromatogram when 5 Bq/L of 90Sr was introduced and measured. Moreover, in the proposed online SPE method, transient signal (= peak) can be observed. It takes about 20 min to analyze a 50 mL sample and about 10 min to analyze a 10 mL sample. It takes only 20 s to identify the peak. Figure 20.8 shows the linearity of the measured 90Sr intensity as a function of its concentration between 5 and 20 Bq/L. The measured 90Sr concentrations were compared with the values measured using an accepted method for radiation measurement—the pico-beta-measurement method. Figure 20.9 shows that the results obtained by the proposed method correlated closely with those obtained by the pico-beta-measurement method in the concentration range from 2 to 100 Bq/L.
Chromatograms of 90Sr and a blank sample (pure water) purified by online solid extraction. When a blank sample (50 mL of pure water) was injected, no peaks were detected on the chromatogram. When 50 mL of 90Sr was injected, an obvious peak with a width of 20 s was detected. When the elution flow rate was 5 mL/min, the preconcentrated phase volume was approximately 0.8 mL. When 50 mL of the sample was injected, the preconcentration factor (volume ratio) was approximately 63 times
The precision of the measurements obtained by ICP-MS analysis also depends on the sample concentration. Figure 20.10 shows the analysis results for samples with 2 and 10 Bq/L (0.4 and 2 pg/L, respectively) of 90Sr in 10 replicates. Even though the 0.4 pg/L concentration is relatively low for ICP-MS, an RSD of 12.6% was achieved, demonstrating the reproducibility of this technique. The measurement of 2 pg/L also showed good reproducibility (RSD of 5.2%). Thus, it can be concluded that, in terms of the method’s sensitivity, reproducibility, and consistency with the conventional method, it is a practical analysis technique.
20.3.5 Addressing the Peaks Associated with the Enriched Stable Sr Isotope
It is known that the measured intensity of the detected elements decreases when a large quantity of the ion is introduced with ICP-MS. The decrease in the temperature of the plasma and the space-charge effect are considered to be the causes of this phenomenon. Basically, coexisting elements are separated while being concentrated and separated in the columns; therefore, the decrease in the measured intensity does not matter when judging the concentrations of minor elements. However, the coexisting stable isotope of Sr is highly concentrated and eluted when measuring 90Sr. This may cause the decrease in the measured intensity. We established an internal standard correction and a signal integration method to solve this problem (Furukawa et al. 2017). Internal standard elements were introduced after the column to obtain a fixed signal intensity. Figure 20.11 shows a variation of the internal standard signals when a high concentration of the stable isotope of Sr was introduced. As the peak of the eluted Sr became larger, the internal standard signal decreased. The ratio of two things between the increased intensity of Sr peak and the decreased intensity of the internal standard signal was calculated as a correction factor for the measured Sr intensity. ICP-MS detects each ion and records its intensity per unit time because each element number is measured continuously using the instrument’s peak-hopping mode. The internal standard correction and signal integration can be used to correct the intensity of the Sr measurements over time to control for the decrease in the whole peak intensity.
Relationship between the peaks of the internal standard (In) and the preconcentrated stable Sr isotope. In the presence of higher concentrations of stable Sr isotope, the sensitivity of the preconcentrated Sr was decreased. As the Sr peak intensity decreased, the peak of internal standard (In) was seen as a negative chromatographic peak
20.3.6 Split-Flow Injection System to Simultaneously Measure the Concentration and Recovery Ratio from a Single Sample (Furukawa and Takagai 2016)
The recovery ratio is defined as the proportion of the elements that are eluted and measured to those that are introduced and concentrated in the resin. The recovery ratio of the column is affected by the other elements coexisting in the sample, physical obstructions (such as the velocity of flow and viscosity), volume differences between samples, and the deterioration of the resin. Thus, to monitor the changes in the recovery ratio, an experiment was designed to introduce a sample and measure its concentration before and after passing through the columns. In other words, more than two measurements must be conducted. The split-flow injection system developed in this study splits samples online before they are injected into the resin such that one portion can be measured directly and the other portion is first concentrated before being analyzed. Thus, the intensity before and after passing through the resin is measured automatically. Because the measured intensities before and after passing through the resin are proportional to the amount of the substance present, the recovery ratio relative to its absolute quantity can be calculated by integrating the measured intensity. In addition, the split-flow injection method can provide a relative recovery ratio without concentrating the sample based on the correlation between the intensities measured before and after passing through the columns.
Figure 20.1 shows a diagram of the split-flow injection. After the sample is input at valve #1, the splitter divides it into two column pathways. The rotational frequency of the peristaltic pump and the inside diameter of the pump tube control the division ratio. When the division ratio is 1:1, the volume of the sample that is concentrated is half that of the sample that is not. To attain greater preconcentration of the sample solution, the split ratio for the direct injection can be increased such that a larger amount of the sample is introduced into the column. Figure 20.12 shows the correlation between the measured intensity before passing through the columns and after concentrating the sample for a standard solution when the division ratio (column injection volume: split volume) was 100:4.
Relationship between the intensities of the split stable Sr isotope and the preconcentrated Sr used to calculate the relative recovery. For the calculation, a linear calibration curve for the split Sr intensity versus the preconcentrated Sr intensity was prepared for a stable Sr standard solution. Based on the line, the relative recovery, R, can be calculated by the following equation given that the split Sr intensity and the preconcentrated Sr peak area have an approximately linear relationship of the form y = aX + b
R = (B)/(C) × 100 = (B)/[a(A) + b] × 100
where a and b were the slope and intercept of the linear line, respectively, A represents the intensity of the split Sr intensity, B is the detected Sr area, and C is the calculated Sr area
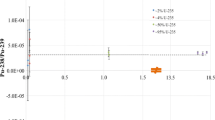
The degree of correlation between the detected intensity of the split sample and the Sr measurement after concentration shows the difference between the recovery ratio of a sample compared to that in the standard solution. Taking the recovery ratio of a standard solution as 100%, the relative recovery ratio of each sample was measured. Figure 20.13 shows the relative recovery ratios of the stable isotope of Sr for which m/z = 88, 86, or 84 (as the signal of 90Sr is hardly detected in the split measurements). The relative recovery ratio of the stable Sr isotope was calculated as a correction factor for the 90Sr measurement to obtain the corrected 90Sr value.
To evaluate the method of split-flow injection, an experiment was conducted with the addition of Ba, known to decrease the Sr recovery ratio. Figure 20.14 shows the measured Sr concentration and the concentration with the corrected relative recovery ratio in the presence of various amounts of Ba and a fixed concentration of Sr. Although the relative recovery ratio of Sr decreased by the addition of Ba, it was confirmed that for the correction of the relative recovery ratio, the use of the split-flow injection method allows the correct concentration (i.e., that without the influence of Ba) to be derived. Therefore, the proposed method is an effective way to correct the recovery ratio. The split measurement does not affect the total measurement time and affects the measurement sensitivity only slightly as the division ratio between the columns and the direct introduction is changed.
Influence of Ba interference on the Sr recovery efficiency and the corrected concentration. The recovery percentage was decreased due to the interference of Ba with the Sr resin. The relative recovery was determined and the corrected concentration was calculated using the split-flow injection. ∆: observed Sr concentration before the correction using the relative recovery efficiency. ○: corrected Sr concentration after the calculation using the relative recovery efficiency
20.4 Conclusion
Here, we demonstrated 90Sr analysis using online SPE/ICP-MS with a split-flow injection method. Several techniques were combined in a single automated system. By using split-flow injection to measure the recovery ratio, the drawback of SPE being affected by changes to the matrix over time was mitigated. This approach can complement the radiation measurement method as an alternative technique for 90Sr measurements and the user may select the proper method depending on the specific application (i.e. rapid measurement and the concentration of 90Sr and stable isotopes etc.). It takes 10–20 min only to complete the 90Sr analysis, requiring more than 2 weeks with the alternative technique. This analysis requires 50 mL of sample and the limit of detection is 0.3 Bq/L (equivalent to about 0.06 pg/L) with an argon-nitrogen mixed gas effect (Furukawa et al. 2018). This ability to measure concentrations as small as a few Bq/L in a small sample makes this technique suitable for a wide-area, multi-point sampling and analysis. Thus, this method can be readily used not only for environmental water analysis but also for applications requiring prompt measurements, such as the analysis of perishable foods.
References
Furukawa M, Takagai Y (2016) Split flow online solid-phase extraction coupled with inductively coupled plasma mass spectrometry system for one-shot data acquisition of quantification and recovery efficiency. Anal Chem 88:9397–9402
Furukawa M, Matsueda M, Takagai Y (2017) Internal standard corrected signal integration method for determination of radioactive strontium by online solid phase extraction/ICP-MS. Bunseki kagaku 66:181–187
Furukawa M, Matsueda M, Takagai Y (2018) Ultrasonic mist generation assist argon–nitrogen mix gas effect on radioactive strontium quantification by online solid-phase extraction with inductively coupled plasma mass spectrometry. Anal Sci 34:471–476
Takagai Y, Furukawa M, Kameo Y, Suzuki K (2014) Sequential inductively coupled plasma quadrupole mass-spectrometric quantification of radioactive strontium-90 incorporating cascade separation steps for radioactive contamination rapid survey. Anal Methods 6:355–362
Takagai Y, Furukawa M, Kameo Y, Matsueda M, Suzuki K (2017) Radioactive strontium measurement using ICP-MS following cascade preconcentration and separation system. Bunseki kagaku 66:223–231
Acknowledgment
The authors would like to thank Dr. Yutaka Kameo, Dr. Kennichiro Ishimori, Mr. Kiwamu Tanaka, and Mr. Makoto Matsueda (Japan Atomic Energy Agency) and Dr. Katsuhiko Suzuki (Japan Agency Marine-Earth Science and Technology). The work was supported by the Ministry of Education, Culture, Sports, Science & Technology in Japan (MEXT), Human Resource Development and Research Program for Decommissioning of Fukushima Daiichi Nuclear Power Station.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Furukawa, M., Takagai, Y. (2019). 90Sr Analysis Using Inductively Coupled Plasma Mass Spectrometry with Split-Flow Injection and Online Solid-Phase Extraction for Multiple Concentration and Separation Steps. In: Nakanishi, T., O`Brien, M., Tanoi, K. (eds) Agricultural Implications of the Fukushima Nuclear Accident (III). Springer, Singapore. https://doi.org/10.1007/978-981-13-3218-0_20
Download citation
DOI: https://doi.org/10.1007/978-981-13-3218-0_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3217-3
Online ISBN: 978-981-13-3218-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)