Abstract
The organisms causing respiratory infections such as influenza are spread in droplets or aerosols or by direct or indirect contact with contaminated surfaces. Certain medical procedures have been termed aerosol generating because they are associated with high or augmented inspiratory and expiratory flows, which can increase microbial dissemination. Invasive ventilation maneuvers and noninvasive ventilation (NIV) fall into that category. We discuss the risk of transmitting these procedures and the strategies for mechanical ventilation in future airborne epidemics with special consideration given to the issue of protecting health care workers (HCWs).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Aerosol-generating procedures
- Noninvasive ventilation
- Personal protective equipment
- Health care workers
1 Introduction
The organisms causing respiratory infections such as influenza are spread in droplets or aerosols or by direct or indirect contact with contaminated surfaces. Certain medical procedures have been termed aerosol generating because they are associated with high or augmented inspiratory and expiratory flows, which can increase microbial dissemination. Invasive ventilation maneuvers and noninvasive ventilation (NIV) fall into that category. We discuss the risk of transmitting these procedures and the strategies for mechanical ventilation in future airborne epidemics with special consideration given to the issue of protecting health care workers (HCWs).
2 Analysis of the Problem
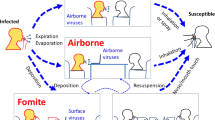
Pathogens in the air are spread on particles or droplets. The solid matter may come from skin, and the droplets may be generated from the upper or lower respiratory tract, mouth, or nose and under such circumstances as vomiting, dripping water taps, and diarrhea. Respiratory droplets, which can carry microorganisms such as bacteria and viruses, constitute a medium for the transmission of infectious diseases. Droplets from the nose and mouth contain bacteria but do not travel >2 m.
The concept of airborne transmission and large droplet transmission is based on droplet size. The classic study of airborne transmission by Wells revealed the relation between droplet size, evaporation, and falling rate. It was determined by studying the evaporation of falling droplets and is referred to as the Wells evaporation-falling curve of droplets. Wells postulated what is now a widely accepted hypothesis of the distinction between droplet size and airborne transmission routes.
Small droplets start to evaporate after release, and thus change their size resulting in droplet nuclei that are sufficiently small to remain suspended in air for a long time and still be infectious. Large droplets (>100 μm) can settle on the ground before they become droplet nuclei [1, 2]. Most respiratory droplets are <100 μm in diameter and evaporate rapidly in the surrounding environment. They become droplet nuclei, which are suspended in the air or are transported away by airflow. The size distribution of droplets is a matter of great debate, but in general various particle sizes are generated: large droplets (>20 μm) that fall directly to the ground or surface; medium-sized particles (5–20 μm), fall at a slower rate or remain temporarily suspended by air currents and evaporate; to become droplet nuclei (aerosol) particles <5 μm in diameter, which remain suspended for longer periods of time [1, 2]. Studies have demonstrated that particles <10 μm in diameter are more likely to cause infection in the lower respiratory tract [3, 4]. The suspension of these droplet nuclei may cause infection over greater distances and increase the duration of infection risk following generation of the initial respiratory aerosol. In addition, the concentration of particles in the secretion and the infectious dose of the pathogen affect the risk of infection. Droplets in the respirable range (~5 μm) may play a significant part in transmission. A few studies have quantified the viral load in droplets or aerosols [5].
An observational study [6] of influenza A and influenza B in exhaled breath showed viral RNA in one-third of infected patients. Also, 99 % of the particles had a diameter <5 μm when sampled during tidal breathing. Although some individuals recover from seasonal or H1N1 influenza after having experienced minimal symptoms, a subgroup of high-risk patients develop complications, including respiratory failure. With the appearance of more pathogenic strains, such as H5N1, respiratory insufficiency may occur in >50 % of those affected. These patients are managed with antiviral therapy and antibiotics for secondary bacterial pneumonia. However, but the mainstay of management is supportive respiratory care, which includes titrated oxygen therapy for hypoxemic patients and ventilatory support for those with respiratory insufficiency [7, 8].
In contrast to the situation regarding severe acute respiratory syndrome (SARS) or tuberculosis prevention in HCWs, little attention has been given to the importance of HCWs personal protective equipment (PPE) (gowns, gloves, masks) for prevention and management of influenza. This situation has arisen because vaccination of HCWs has been shown to reduce or prevent nosocomial transmission. It seems prudent for nonvaccinated workers to wear N-95 masks, particularly during high-risk procedures or with very ill patients. There is limited evidence that upper-air ultraviolet light is effective in reducing influenza transmission rates.
Some medical procedures have been termed aerosol-generating procedures (AGPs) as their most common feature is that they are associated with high or augmented inspiratory and expiratory tidal flows, which may increase viral aerosol dissemination. The list of AGPs [5] include bronchoscopy, airway intubation, and invasive ventilation maneuvers such as open suctioning, cardiopulmonary resuscitation, NIV, and continuous positive airway pressure (CPAP) therapy, high-frequency oscillation ventilation, and induction of sputum. Certain other procedures, such as delivery of nebulized medication therapy and high-flow O2, are considered possible aerosol generators but of lesser infective risk. There is an association between some of these AGPs and an increased incidence of SARS in HCWs with super-spreading events on the wards [9].
Much of the evidence for the link between AGPs and increased transmission of respiratory viral infection was generated during the SARS epidemic. In Toronto, China, and Singapore, HCWs constituted approximately 20 % of the critical care cases. Infection rates were higher in doctors and nurses carrying out endotracheal intubation [relative risk (RR) 13.29, 95 % confidence interval (CI) 2.99–54.04, p = 0.03], and nurses caring for SARS patients receiving NIV may have been at increased risk (RR 2.23), but these findings did not reach significance [95 % confidence interval (CI) 0.25–21.76, p = 0.5] [10]. In a case–control study of the dissemination of SARS from an index case to other patients on the same ward, Yu et al. [9] showed an increased risk associated with the index patient requiring oxygen or bilevel NIV. Case reports [11, 12] have also linked transmission of infection to nebulizer use in the index patient. However, patient variables are also important factors to consider: Sicker patients have a higher viral load and are more likely to require oxygen and ventilator support, and those with underlying asthma require nebulizer therapy and cough more because of airway hyperreactivity. Both settings increase the risk of aerosol transmission.
There is additional evidence concerning AGPs and the risk they present to HCWs. Experimental studies that have investigated airflows around oxygen masks and during NIV [13–18]. These studies used human simulator models or normal subjects mimicking respiratory distress. Hui et al. [16] examined smoke particle dispersion from the lungs of a human simulation model receiving oxygen therapy, frequently used in the treatment of patients with respiratory failure. The authors found that a jet plume of smoke could be generated from exhaust holes up to 0.45 m from the mask. Although this model provides a visual image of smoke aerosol behavior, and the possible zone of transmission risk, it is not necessarily representative of the behavior of a respiratory aerosol and infectious particles contained therein.
Two similar studies were carried out on oxygen masks. One indicates that oxygen mask usage might contribute to droplet-respiratory transmission of SARS [14]. The other observed a visible range of the smoke plume of 0.08–0.40 m depending on the type and flow rate of the mask used [19].
Simonds et al. [20] evaluated the characteristics of droplet/aerosol dispersion around delivery systems during NIV, O2, nebulizer treatment, and chest physiotherapy by measuring the droplet size, geographical distribution of droplets, decay in droplets over the time after the intervention was discontinued, and the impact of modifying the NIV circuit in clinical practice. Three groups of patients were studied: normal control subjects; subjects with coryzal symptoms; adults with chronic lung disease who were admitted with an infective exacerbation. Each group received O2, NIV using a vented mask system, and a modified circuit with a nonvented mask and an exhalation filter. All received nebulized saline and a period of standardized chest physiotherapy. Droplet counts in mean diameter sizes ranging from 0.3 to >10.0 μm were measured with a counter placed adjacent to the face and at 1 m distance from the patient at the height of the nose/mouth of an average HCW. NIV using a vented mask produce large droplets (>10 μm) in patients (p = 0.042) and coryzal subjects (p = 0.044) compared with baseline values but not in normal controls (p = 0.379). This increase in large droplets was not seen using the NIV circuit modification. Chest physiotherapy produced droplets predominantly >10 μm (p = 0.003), with the droplet count (as in the NIV patients) falling significantly by 1 m. O2 did not increase the droplet count in any size range. Nebulized saline delivered droplets in the small and medium size aerosol/droplet range, in keeping with the specified performance characteristics of the device, but did not increase the large-droplet count. Preliminary analyses suggest that droplet counts fall to within a baseline range within 20–40 min of discontinuing the NIV and chest physiotherapy.
In conclusion, NIV and chest physiotherapy are droplet- (not aerosol-) generating procedures, producing droplets >10 μm. Because of their large mass, most fall on local surfaces within 1 m. The only device producing an aerosol was the nebulizer. The output profile is consistent with nebulizer characteristics rather than dissemination of large droplets from patients. These findings suggest that HCWs who are providing NIV and chest physiotherapy and are working within 1 m of an infected patient should have a high level of respiratory protection. Infection control measures designed to limit aerosol spread (e.g., negative-pressure rooms) may have less relevance.
Tran et al. [21] systematically reviewed the literature regarding the risk of transmitting acute respiratory infections to HCWs exposed to patients undergoing an AGP compared with the risk of transmission to HCWs caring for patients not undergoing an AGP. The outcome of interest was the risk of acute respiratory infection. They identified five case–control and five retrospective cohort studies that evaluated transmission of SARS to HCWs. The procedures reported to present an increased risk of transmission included tracheal intubation [n = 4, cohort: odds ratio (OR) 6.6 (2.3–18.9); n = 4, case–control study: OR 6.6 (4.1–10.6)] and NIV [n = 2, cohort: OR 3.1 (1.4–6.8)]; tracheotomy [n = 1, case-control: OR 4.2 (1.5–11.5)]; and manual ventilation before intubation [n = 1, cohort: OR 2.8 (1.3–6.4)]. Other intubation-associated procedures, endotracheal aspiration, suction of body fluids, bronchoscopy, nebulizer treatment, administration of O2, high-flow O2, manipulation of O2 masks or bilevel positive airway pressure (Bi-PAP) masks, defibrillation, chest compression, insertion of a nasogastric tube, and collection of sputum were not significant. These findings suggest that some procedures potentially capable of generating aerosols have been associated with increased risk of SARS transmission to HCWs or were a risk factor for transmission. The most consistent association across multiple studies was tracheal intubation. The results of this report should not be generalized to all acute respiratory infections because the evidence available is strictly limited to SARS.
Noninvasive ventilation and continuous positive airway pressure are likely to play a minor role in the management of moderate to severe acute lung injury caused by influenza or secondary bacterial pneumonia, or in patients with multisystem failure. However, NIV was used successfully in some SARS cases [22]. There is also potential for NIV to reduce the need for intubation in patients with influenza pneumonia or chronic respiratory disease, facilitate extubation, and widen the provision of ventilator support outside the intensive care unit (ICU). It may also be used as ventilator care in patients with chronic obstructive pulmonary disease, congestive cardiac failure, and other serious co-morbidities. NIV is sometimes used to palliate symptoms in those with end-stage disease in whom ICU admission is not indicated [23]. These indications should be set against the risk of droplet dissemination during the delivery of NIV. Despite the study of Simonds et al. [20], which indicated that NIV generates large droplets adjacent to the patient that fall significantly at 1 m from the patient, and that adding a circuit using a nonvented mask plus a filtered exhalate reduces the number of large droplets produced, there is still concern about dispersion of infectious particles. Nevertheless, in a Hong Kong hospital where more than 20 patients were placed on noninvasive positive ventilation, all HCWs on the ward performed meticulous infection-control procedures and used PPE. Despite the intense exposure, none became infected with SARS [24]. Patient selection is important for NIV as it has not been shown to improve the mortality rate among patients with acute respiratory distress syndrome (ARDS) and may be not suitable for patients in whom short-term improvement is not expected [25, 26].
Protection of the HCW during mechanical ventilation includes isolation of infected patients, use of PPE, and strict hand hygiene by all. The World Health Organization and the Centers for Disease Control and Prevention have issued guidelines that recommend the use of standard, contact, and airborne protection, including respirators of N-95 standard or higher, which filter at least 95 % of particles that are ≥1 mm with <10 % face seal air leak. These filters not only protect against virus-transmitted diseases but also against tuberculosis (TB), filtering at least 95 % of the 3- to 5-mm TB bacilli out of the air inhaled by HCWs. The need for N-95 masks depends on the mode of transmission. If transmission is solely by droplet, face shields, eye protection, and surgical masks are adequate. However, if transmission is airborne, N-95 masks should be used. As reviewed earlier, there is evidence that airborne transmission of SARS occurred, at least from the super-spreaders or during aerosol-generating activities such as intubation or suctioning. Knowing that super-spreaders are identified only in retrospect, it may be prudent for workers to wear N-95 masks at all times.
Standard personal protective equipment includes N-95 masks, gloves, gowns, caps, and face shields or goggles [26, 27]. All staff should be mask fit-tested to ensure adequate seal. When performing high-risk procedures such as intubation, bag-mask ventilation, or bronchoscopy, protection should be enhanced with powered air-purifying respirators. Also, the HCW should be aware that these procedures have been associated with increased risk of infection transmission and should upgrade to airborne infection control precautions [28] (Table 34.1).
In view of the high risk of disease transmission during endotracheal intubation, airway management protocols have been proposed: Early intubation should be performed, preferably in the ICU, rather than performing a crash intubation on the ward. Adequate sedation and neuromuscular blockade is recommended during intubation to minimize cough and dispersion of respiratory secretions. Finally, the procedure should be performed by the most experienced person available to minimize the dispersal of infectious particles and reduce the number of individuals exposed during intubation [29]. Measures to minimize respiratory droplet transmission include using in-line suctioning to maintain the ventilator circuit as a closed system. Humidification should be done via heat-moisture exchangers with viral-bacterial filter properties rather than heated humidifiers. Each ventilator should have two filters—one between the inspiratory port and ventilator circuit and the other between the expiratory port and ventilator circuit—to provide additional protection from exhaust gases and minimize ventilator contamination [26].
Other general recommendations include using a unidirectional/displacement ventilation system for a single patient room. It should not be used in a multi-bed ward where the potentially aerosol-transmitted infection patient source is unknown as this ventilation system may unintentionally disseminate the infection throughout the ward to other patients. Hence, the situation where such a ventilation system is used needs to be considered carefully. Even though an ideal isolation unit is fitted with a negative-pressure system and sliding glass doors (to reduce airflow generated by traditional hinged doors), it is possibly the movement of people in and out of the room that produces the most significant airflow. Of course, it is impossible to prevent such movement in a health care facility, but reducing the number of times the room is entered or exited can reduce the volume of potentially infected air exchanged across the doorway.
An essential component of an infection-control strategy is staff training. Clear management protocols must implemented, including the use of PPE, monitoring staff health, quarantining staff, transport of patients, transfer to the ICU, airway management, aerosol generating procedures, environment and equipment disinfection, and visitation policies.
The health care environment could be an important reservoir for viruses, bacteria, and fungi during outbreaks, given their proven ability to survive on surfaces and to become airborne. Changes in temperature and humidity in hospitals could have relevance for the viability of microorganisms and their spread to other patients. Adequate ventilation is necessary to dilute the airborne microbial load. Heat and humidity need to be controlled. It is recommended that upper and lower limits for temperature and humidity be specified according to the outbreak pathogen and that air changes at the patient level be tested regularly, especially after any restriction to airflow.
It is important for intensive care providers to be prepared to meet the challenge of large-scale airborne epidemics causing mass casualty respiratory failure. Previous outbreaks have exposed the vulnerability of HCWs and highlighted the importance of establishing stringent infection control and crisis management protocols. There should be an established lung-protective, low tidal volume strategy for treating patients with acute lung injury or ARDS who require mechanical ventilation. The use of NIV remains controversial. Current infection-control policies that limit or prohibit the use of NIV as a high-risk intervention are based largely on supposition [30]. Standard contact and airborne precautions should be instituted in the ICU, with special care taken when aerosol-generating procedures are performed (Table 34.2).
Key Major Recommendations
-
The evidence regarding the risk of transmission during NIV is conflicting and unclear.
-
There have been reports of NIV being effective in treating patients during an epidemic, reducing the need for intubation.
-
NIV should be used especially in a pandemic scenario when the demand for mechanical ventilation support is overwhelming.
-
Health care workers performing NIV during an airborne epidemic should use standard, contact, and airborne protection, including respirators of N-95 standard or higher.
References
Eames I, Tang JW, Li Y, Wilson P. Airbone transmission of disease in hospitals. J R Soc Interface. 2009;6:S697–702.
Aliabadi AA, Rogak SN, Barlett KH, Green S. Preventing airbone disease transmission: review of methods for ventilation design in health care facilities. Adv Preven Med. 2011;2011:1–21.
Brigdes CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–101.
Beggs CB. The airbone transmission of infection in hospital buildings: fact or fiction? Indoor Built Environ. 2003;12:9–18.
Davies A, Thomson G, Walker J, Bennet A. A review of the risks and disease transmission associated with aerosol generating medical procedures. J Infect Prev. 2009;10(4):122–6.
Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chang KH, et al. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3:e2691.
ANZIC The Influenza Investigators. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Eng J Med. 2009;361:1925–34.
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalised patients with H1N1 influenza in the United States. N Eng J Med. 2009;361:1935–44.
Yu IT, Xie Z, Tsoi K. Why did outbreaks of severe acute respiratory syndrome occur in some hospitals wards an not others? Clin Infect Dis. 2007;44:1017–25.
Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198–202.
Lee N, Hui DS, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Eng J Med. 2003;248:1986–94.
Wong RS, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10:339–41.
Somogyi R, Vesely AE, Azami T, Fisher J, Correia J, Fowler RA. Dispersal of respiratory droplets with open vs closed oxygen delivery masks. Implications for the transmission of severe acute respiratory syndrome. Chest. 2004;124(3):1155–7.
Hui DS, Hall SD, Chan MTV, Chow BK, Tsou JT, et al. Noninvasive positive-pressure ventilation. An experimental model to assess air and particle dispersion. Chest. 2006;130:730–40.
Hui DS, Ip M, Tang JW, Wong ALN, Chan MTV, et al. Airflows around oxygen mask. A potential source of infection? Chest. 2006;130:822–6.
Hui DS, Hall SD, Chan MTV, Chow BK, Gin T, et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132:540–6.
Hui DS, Chow BK, Ng SS, Chu LCY, Hall SD, Gin T, et al. Exhaled air dispersion distances during noninvasive ventilation via different respironics face masks. Chest. 2009;136:998–1005.
Hui DS, Chow BK, Chu LCY, Ng SS, Hall SD, Gin T, Chan MT. Exhales air an aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135:648–54.
Ip M, Tang JW, Hui DS, Wong AL, Chan MT, et al. Airflow an droplets spreading around oxygen mask: simulation model for infection control research. Am J Infect Control. 2007;35:684–9.
Simonds AK, Hanak A, Chatwin M, Morrell MJ, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebulizer treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airbone infections. Health Technol Assess. 2010;14(46):131–72.
Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797.1–8.
Cheung Thomas MT, Yam Loretta YC, So Loretta KY, Lau Arthur CW, Poon E, Kong Bernard MH, Yung Raymond WH. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–50.
Esquinas A. Noninvasive mechanical ventilation. Theory, equipment and clinical applications. Berlin/Heidelberg: Springer; 2010. p. 1–397.
Arabi Y, Gomersall CD, Ahmed QA, et al. The critical ill avian influenza A (H5 N1) patient. Crit Care Med. 2007;35:1397–403.
Gruber PC, Gomersall CD, Joynt GM. Avian influenza (H5 N1): implications for intensive care. Intensive Care Med. 2006;32:823–929.
Phua GC, Govert J. Mechanical ventilation in an airbone epidemic. Clin Chest Med. 2008;29:323–8.
Manuell ME, Co MDT, Ellison III RT. Pandemic influenza: implications for preparation and delivery of critical care services. J Intensive Care. 2011;26:347–67.
Hui DS, Lee N, Chan PKS. Clinical management of pandemic 2009 influenza A (H1N1). Chest. 2010;137(4):916–25.
Caputo KM, Byrick R, Chapman MG, et al. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122–9.
McCraken J. Should noninvasive ventilation be considered a high-risk procedure during an epidemic? CMAJ. 2009;181:663–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Flores, M.V., Cohen, M. (2014). Preventing Airborne Disease Transmission: Implications for Patients During Mechanical Ventilation. In: Esquinas, A. (eds) Noninvasive Ventilation in High-Risk Infections and Mass Casualty Events. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1496-4_34
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1496-4_34
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1495-7
Online ISBN: 978-3-7091-1496-4
eBook Packages: MedicineMedicine (R0)