Abstract
Neurons in the ischemic penumbra or peri-infarct zone may undergo delayed cell death which called programmed cell death (PCD) and thus they are potentially recoverable for some time after the onset of stroke. There were three major morphologies of PCD in the cerebral ischemic injury, including apoptosis, autophagy and programmed necrosis (also known as necroptosis). In this review we will discuss the characteristics, molecular mechanism of each PCD mode and their role in cerebral ischemia and reperfusion injury (CIRI), furthermore crosstalk between various modes of PCD is also dicussed.
Similar content being viewed by others
Keywords
- Programmed cell death
- Apoptosis
- Autophagy
- Programmed necrosis
- Necroptosis
- Cerebral ischemia and reperfusion injury
1 Introduction
When blood flow to the brain is interrupted, cells undergo a series of molecular events which include excitotoxicity, mitochondrial dysfunction, acidotoxicity, ionic imbalance, oxidative stress and inflammation. These molecular events can lead to cell death and irreversible tissue injury [1, 2]. The fate of brain cells following cerebral ischemia depends upon the severity of the insult and vulnerability of the neurons. The severity of ischemia depends on the extent of cerebral blood flow (CBF) reduction that determines the degree and deprivation of oxygen and glucose from the cells, however a particular threshold do exist for various kinds of pathophysiologic tissue events. Moreover, the high sensitivity of the brain to blood flow changes and dependence on continuous blood flow are critical factors that make the brain particularly more vulnerable to ischemia. Under physiological conditions, the normal CBF is maintained around 50–60 mL/100 g/min but during cerebral ischemia due to declining CBF, the ripples of damage spread from the center towards the periphery forming a gradient in such a way that maximum damage (infarction) is at the center (core). The CBF in this region falls to <7 mL/100 g/min and within minutes of a focal ischemic stroke occurring, the core of brain tissue exposed to the most dramatic blood flow reduction is fatally injured and subsequently undergoes necrotic cell death. The ischemic core is surrounded by region of moderate ischemic zone called ischemic penumbra (IP), with a CBF ranging from 7 to 17 mL/100 g/min [3], which remains metabolically active but electrically silent [4]. The ischemic penumbra region may comprise as much as half the total lesion volume during the initial stages of ischemia, and represents the region in which there is opportunity for salvage via poststroke therapy. Recent research has revealed that many neurons in the ischemic penumbra or peri-infarct zone may undergo delayed cell death, and thus they are potentially recoverable for some time after the onset of stroke.
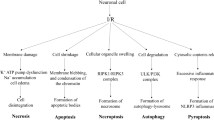
This delayed cell death modal was usually called programmed cell death (PCD), which was different from the necrotic cell death that has been considered merely as an accidental uncontrolled form of cell death. PCD is defined as regulated cell death mediated by an intracellular program, which is a basic biological phenomenon that plays an important role during development, preservation of tissue homeostasis, and elimination of damaged cells. There were three major morphologies of programmed cell death in the ischemic injury, including type I, apoptosis; type II, autophagy; and type III, programmed necrosis (known as necroptosis) [5, 6].
Type I—apoptotic cell death—acts as part of a quality control and repair mechanism by elimination of unwanted, genetically damaged, or senescent cells, and as such is critically important for the development of organisms. Highly conserved in both plants and animals, it is also the cell death mechanism best characterised at both genetic and biochemical levels [7]. Type II—autophagic cell death—is a catabolic process conserved among all eukaryotes from yeast to mammals; it is a mechanism by which organelles are removed. Autophagic cell death is the primary degradation mechanism for long lived proteins, and thus maintains quality control for proteins and organelles to enhance survival under conditions of scarcity or starvation [8]. Type III—programmed necrosis [6, 9]—appears as a distinct entity, not by exclusive engagement of selected effectors, but rather, by combinatorial use of the effectors shared with other cell death outcomes.
PCD displays several cellular phenotypes affecting various intracellular organelles and membranes, and the cell nucleus. For example, the well characterised processes of cytoplasmic and chromatin condensation, nuclear fragmentation, membrane blebbing, and formation of membrane bound apoptotic bodies are part of apoptosis. Autophagy involves the formation of a double membrane vesicle which encapsulates cytoplasm and organelles, and fuses with lysosomes, thus resulting in the degradation of the vesicle contents. Programmed necrosis is characterised by the presence of swelling organelles followed by the appearance of “empty” spaces in the cytoplasm that merge and make connections with the extracellular space. The plasma membrane is fragmented, but the nucleus is relatively preserved (Fig. 5.1).
Morphological (electron microscope) features of autophagic, apoptotic and necrotic cells. (a) Normal, (b) autophagic, (c) apoptotic (d) and necrotic cells. Whereas the morphologic features of apoptosis are well defined, the distinction between necrotic and autophagic death is less clear. The bioenergetic catastrophe that culminates in cellular necrosis also stimulates autophagy as the cell tries to correct the decline in ATP levels by catabolizing its constituent molecules. Thus, vacuolation of the cytoplasm is observed in both autophagic cells (b) and in cells stimulated to undergo programmed necrosis (d). By contrast, ATP levels are maintained in normal (a) and apoptotic cells (c) consistent with the limited number of autophagic vacuoles in their cytoplasm. The scale bar represents 1 mm (From “Death by design: apoptosis, necrosis and autophagy” by Aimee L Edinger and Craig B Thompson [10])
Up to now, the only available therapeutic strategy for ischemic stroke is to reopen an occluded artery by thrombolytic therapy to restore perfusion to the ischemic area during the first few hours, procedure which in itself can sometimes induce secondary damage, so this delayed cell death modalities “programmed cell death” after CIRI provide us a extended therapeutic time window, which is an important research direction in the ischemic stroke study.
2 Apoptotic Cell Death in CIRI
The morphology of apoptotic cells is characterized by vacuoles containing cytoplasm and intact organelles which are named apoptotic bodies. Before the loss of cell membrane integrity, the dying cell is gradually shrinking and absorbed by phagocytic uptake. To date, research indicates that there are two main apoptotic pathways: the intrinsic and extrinsic pathways [11, 12]. The former is also called the mitochondrial pathway because the disruption of mitochondria is pivotal in the process, which leads to the release of the cytochrome C and the downstream activation of caspases. The other pathway, referred as the extrinsic pathway, receptors can be activated by specific ligands that bind to cell surface death receptors.
There are lots of other factors influencing post-stroke apoptosis, including age and gender [13, 14]. It is said that immature brains are more sensitive to the induction of apoptosis because caspase-3 is activated much more in immature brains than in those of adults [13]. Besides, sex hormone exposure may lead to higher risk of cerebral ischemia for women. The pathways of cell death differ in sexual dimorphism, as caspase-dependent pathway is more involved in female whereas AIF translocation is more important in males [14].
It is a common physiological death mechanism in ischemic stroke; but it also causes further impairment under certain pathological conditions. Energetic stress is the consequence of cerebral ischemia, and then reperfusion is accompanied by abrupt ionic shifts and considerable oxidative stress. During above physiopathologic process, apoptosis plays a key role of the neurons.
2.1 Molecular Biology Mechanism of Apoptosis After Ischemic Stroke
2.1.1 Molecules Related to Apoptosis of Neurons
2.1.1.1 Caspase Family
There are totally 14 caspase proteins identified by researchers, and among them at least eight proteins participate in the cell apoptosis. Caspase related to apoptosis can be classified into two types, the trigger and the executor. Caspase-8, Caspase-9 and Caspase-10 belong to the triggers while Caspase-3 and Caspase-7 are the executors [15]. Caspase-3 has been identified as a key mediator of apoptosis in animal models of ischemic stroke [12]. Activation of Caspase 3 requires assembly of a large multimeric complex comprising Caspase 9, APAF1, and cytochrome c. Caspase-3 cleaves many substrate proteins, including poly (ADP-ribose) polymerase (PARP) [16]. PARP inactivation after cleavage by caspase-3 leads to DNA injury and subsequently to apoptotic cell death [17]. In brief, Caspase-3 and Caspase-7 are the main participate and executor when apoptosis is activated after ischemic brain injury.
Albeit the underlying mechanism is consistent, the severity of ischemia, temporal and spatial heterogeneity may influence the specific condition of neuronal cell death. In the early stages of cerebral infarction, caspase-8 and caspase-1 are involved in the early apoptosis, contributing to the core. However, caspase-9 is related to the secondary expansion of the lesion in the penumbral area [18].
2.1.1.2 B-Cell Leukemia/Lymphoma 2 (Bcl-2) Family
The B-cell leukemia/lymphoma 2 (Bcl-2) family has the role of maintaining the integrity of the mitochondrial membrane. It has three subfamilies according to the molecular structure [19,20,21]. The first subtype is antiapoptotic protein, including Bcl-2, Bcl-xl (B-cell lymphoma-extra large) and Bcl-w. Proapoptotic protein is the second subtype, for instance, Bax (Bcl-2-associated X protein) and Bak (Bcl-2 homologous antagonist killer). The last is Bcl-2 homology domains 3 (BH3) domain protein including Bad (Bcl-2-associated death promoter), Bid (BH3 interacting-domain death agonist), Bim (Bcl-2-interacting mediator of cell death), Noxa and p53 [22]. Cerebral ischemia and reperfusion lead to intracellular stress originating from the mitochondria, the endoplasmic reticulum and the nucleus. The proteins from Bcl-2 family are sensitive to these stress factors after cerebrovascular events.
2.1.1.3 Tumor Necrosis Factor Receptor (TNFR) Superfamily
The Tumor necrosis factor receptor (TNFR) superfamily includes Fas and TNFR1 [23]. Fas is also called CD95 or Apo1. The Fas ligand (FasL) is a homotrimer, constituting microaggregate on the surface of cells. Caspase-8 is activated by death-inducing signaling complex (DISC), of which Fas is an important part.
2.1.1.4 Other Molecules
Besides, there are still other potential molecules that participate in the post-stroke apoptosis. Nuclear factor-Y transcription factor (NF-YC) [24], Secretory phospholipase A2 (sPLA2) [25], Bim [26], Numb [27] have been suggested to be correlated with apoptosis of neurons by experiments.
2.2 Pathways Related to Apoptosis of CIRI
2.2.1 Intrinsic Pathway
The stimulation by glutamate of N-methyl-d-aspartate (NMDA), amino-3-hydroxy-5-methyl-isoxazolpropionic acid (AMPA) receptors, or acid-sensing ion channels (ASICs) causes high-level intracellular calcium after cerebral ischemia [28, 29]. Then, the increased cytosolic calcium activates calpains and induces the cleavage of Bid. The truncated Bid (tBid) interacts with apoptotic proteins such as Bad and Bax at the mitochondrial membrane, which is called heterodimerization [30]. On the other hand, antiapoptotic Bcl-2 interacts with apoptotic proteins and neutralizes their effects. The above process involved Bax and Bcl-2 is the critical event in the mitochondrial-mediated pathway [31]. Mitochondrial transition pores (MTP) are opened after the heterodimerization. Cytochrome c (Cytc) is released from the pores into the cytosol. Then an apoptosome is constituted by Cytc, procaspase-9 and apoptotic protein-activating factor-1 (Apaf-1) [31]. The apoptosome plays the role of activating caspase family. Activated caspase-3 by caspase-9 exert the ultimate effect of nDNA damage and apoptosis through cleaving nDNA repair enzymes such as poly ADP-ribose polymerase (PARP). By contrast, apoptosis-inducing factor (AIF) mediates cell death by a caspase-independent method, which is also released from the pores and translocates rapidly to the nucleus. Phosphorylation and activation of p53 can also mediates the neuronal apoptosis by damaging DNA [32, 33]. Noticeably, secondary reperfusion injury carrying superoxide anions, can also cause DNA damage.
2.2.2 Extrinsic Pathway
There is considerable evidence from animal studies indicating that brain ischemia triggers the extrinsic apoptotic signaling cascade. Due to the initiating effect of death receptors on the plasma member, the extrinsic pathway is also named receptor-mediated pathway. The extracellular Fas ligand (FasL) binds to Fas death receptors (FasR), which triggers the recruitment of the Fas-associated death domain protein (FADD) [31]. FADD binds to procaspase-8 to create a death-inducing signaling complex (DISC), which activates caspase-8 [34]. Activated caspase-8 either mediates cleavage of Bid to truncated Bid (tBid), which integrates the different death pathways at the mitochondrial checkpoint of apoptosis, or directly activates caspase-3. At the mitochondrial membrane tBid interacts with Bax, which is usually neutralized by antiapoptotic B-cell leukemia/lymphoma 2 (Bcl-2) family proteins Bcl-2 or Bcl-xL. Dimerization of tBid and Bax leads to the opening of mitochondrial transition pores (MTP), thereby releasing cytochrome c (Cytc), which execute caspase 3-dependent cell death.
2.3 Significance of Apoptosis in CIRI
The molecular mechanisms of apoptosis after stroke enlighten the exploration of neuroprotective agents. Ischemic preconditioning in animals triggers activation of caspase-3 downstream and upstream of its target caspase-activated DNase (CAD) to prevent neuronal death [35]. Furthermore, enhanced formation of Apaf-1/caspase-9 complex is observed in the rat hippocampus 8–24 h after ischemia [36, 37]. Cao et al. have cloned a rat gene product, a specific Apaf-1 inhibitor of the Apaf-1/caspase-9 pathway that can be neuroprotective in CIRI [12, 35]. Therefore, Apaf-1 signaling pathway may be a legitimate therapeutic target for the treatment of ischemic brain injury [38]. Fas/FasL system acts as apoptosis inducer and triggers pro-inflammatory cytokine production, while the hematopoietic growth factor, erythropoietin (EPO) inhibits apoptosis and protects from ischemic neuronal damage [39]. These findings indicate that death receptors are critically engaged in the apoptosis induction after ischemia in the adult brain and that their suppression may improve the neuronal survival after ischemic injury [12, 40]. FTY720, another antiapoptotic agent, successfully decreased cleaved Caspase-3 expression by activation of sphingosine 1-phosphate-1 in rats after cerebral artery occlusion [41]. In global cerebral ischemia in the gerbils, treatment with a purified medicinal herb called baicalin remarkably promoted the expression of BDNF and inhibited the expression of caspase-3 at mRNA and protein levels [42]. Additionally, it is reported that different concentrations of normobaric oxygen can inhibit the apoptotic pathway by reducing caspase-3 and -9 expression, thereby promoting neurological functional recovery after CIRI [43]. These are various neuroprotective agents on the animal models and they are potential therapeutic targets in future clinical pharmacological research.
3 Necroptosis in CIRI
Necrosis was classified as non-programmed necrotic death previously which has been described as a response of extreme stress. However, in recent years, there is strong evidence to confirm that part of necrosis also contained program control, therefore proposed new concept as programmed necrosis or named necroptosis. Necroptosis are all classified as programmed cell death based on morphological and biochemical features [6, 44]. This phenomenon was observed in the ischemic stroke model.
3.1 Signal Pathway of Necroptosis
Caspase inhibition cannot blocked tumor necrosis factor (TNF) induced cell death completely, but rather switch to cell fate to necrotic death signal pathway like apoptosis [45, 46]. TNFα is the major trigger of necroptosis, which has capable of initiating caspase-8-dependent apoptosis and RIPK1 kinase-dependent necroptosis [47]. Caspase-8 plays a critical regulatory role in the switch. When FADD-caspase-8-FLIP complex functions inhibited, the cell death pathway switches from apoptosis to typical necroptosis features [48,49,50,51].
TNF-α induced necroptosis is the mostly intensively investigated. TNF receptor 1 (TNFR1) ligation leads to the recruitment of TRADD, TRAF2 and cIAP1/2, which is named as complex I [52]. The complex I activating death-inducing TNFR1 complex II via cylindromatosis (CYLD) [53]. In necrotic signal pathway, receptor-interacting kinase 1 (RIP1 or RIPK1) was the first molecule identified as the core components of the necroptotic machinery [54]. When RIPK1 and RIPK3 phosphorylated, then formed a necrosome through their homotypic interaction motif (RHIM) domains, and activates their kinase activities [55]. This RIPK1–RIPK3 interacts with mixed-lineage kinase domain-like (MLKL) phosphorylation [56]. Downstream of the necrosome are two splice variants of PGAM5, PGAM5S and PGAM5L. PGAM5L binds to the necrosome is not affected by the presence of the necrosis inhibitor necrosulfonamide (NSA). However, the binding of PGAM5S is blocked by NSA. Furthermore, mitochondrial fragmentation caused by the mitochondrial phosphatase PGAM5S recruited the mitochondrial fission factor Drp1 may up-regulate ROS generation [57].
3.2 Necroptosis in Cerebral Ischemia Disease
Necroptosis delayed mouse ischemic brain injury in the absence of apoptotic signaling [58]. In hippocampal neurons oxygen-glucose deprivation (OGD) models RIP3 mRNA and protein levels upregulation nevertheless caspase-8 mRNA downregulation. Similar to RIP3, RIP1 protein level was correlated with the activation of neuronal death. Consistent with the classical procedural necroptosis cellular pathways, ischemic injury upregulated RIP1-RIP3 expression and decreased the caspase-8 expression, which may be available afterwards for activation of necroptotic signaling [59].
Global brain ischemia and reperfusion (I/R) injury is another form of brain cell injury, which the hippocampal CA1 layer is especially vulnerable [60]. As a marker of necroptosis, RIP3 upregulated and transferred into nucleus after cerebral ischemia and reperfusion injury. RIP1–RIP3 complex is necessary for TNF induced necropoptosis in cell cytosol. ATP depletion is one of the results of the mitochondrial permeability transition pore (mPTP) leads to mitochondrial swelling. CypD as a gatekeeper of mPTP, alleviated the levels of RIP1 and RIP3, which mediated mPTP opening may contribute to not only apoptosis but also necroptotic cell death in cerebral I/R injury [61]. RIP3 was activated after I/R injury, and then interacts with AIF in the cytoplasm. The nuclear translocation of AIF and RIP3 is critical to neuronal necropoptosis, and the nuclear translocation of AIF may be RIP3-dependent [51]. AIF is the mediating molecule that links caspase-independent PCD with the necroptotic pathway.
It was observed that nerve cell necrosis occurred following focal middle carotid artery occlusion/reperfusion (MCAO/R) ischemic stroke model. TNFR1 and RIP3 were positively expressed and significantly increased following the volume of cerebral infarction post-reperfusion. Pre-administration with Z-VAD-FMK (zVAD) significantly increased the protein level of RIP3 [62]. In addition to phosphorylation modification, RIP3 S-nitrosylation in ischemia and reperfusion paralleled with elevated phosphorylation. It means RIP3 could be regulated by its S-nitrosylation triggered by NMDAR-dependent nNOS activation [63].
3.3 The Regulation of Necroptosis in Cerebral Ischemic Model
The classic inhibitor is a small molecule compound NSA, which did not block necrosis-induced RIP1 and RIP3 interactions, it blocks necroptosis downstream of RIP3 activation. In human glioblastoma cells, NSA switch from necrosis to apoptosis in edelfosine-treated [64].
In the field of cerebral ischemia, Necrostatin-1 (NEC-1) is another inhibitor of necroptosis has been shown to ameliorate tissue damage in ischemic brain injury animal models [58]. NEC-1 has a selective primary cellular target responsible for the death domain receptor-associated adaptor kinase RIP1 activity [65, 66]. It not only inhibited the expression of RIP1, prevented upregulation and nuclear translocation of RIP3, but also decrease cathepsin-B releasing in globe cerebral ischemic model. CA074-me and 3-methyladenine (3-MA), as autophagy inhibitors [67], were used to determine whether beneficial for global cerebral ischemia in the process of necroptosis signal pathways. The mechanism of 3-MA is inhibiting the nuclear translocation and co-localization of RIP3 and AIF. As the nuclear translocation of RIP3-AIF complex is critical to ischemic neuronal DNA degradation and necroptosis [51] (Fig. 5.2). Beside this, CA074-me almost completely hampered the loss of mitochondrial membrane depolarization, phosphatidylserine (PS) translocation, and plasma membrane rupture [68].
RIP1 and RIP3 are activated (phosphorylated) and combine with each other after CIRI. AIF is released from mitochondria and combines with RIP3 (perhaps phosphorylated RIP3) to form RIP3-AIF complexes. The RIP3-AIF complexes translocate into the nucleus resulting in chromatin condensation and DNA degradation, and then the neurons are triggered to undergo programmed necrosis. All of these changes after I/R injury are inhibited by pre-treatment with Nec-1 and 3-MA, except for the release of AIF from mitochondria in the 3-MA pre-treatment group. In neurons, the findings that caspase-8 expression was undetectable and caspase-3 was not activated indicate that caspase-dependent apoptosis is not involved in this process. Another necroptosis pathway in the cytoplasm induced by RIP1-RIP3-MLKL complexes, described by others, may also participate in this process
4 Autophagic Cell Death in CIRI
Autophagy is the process by which a membrane engulfs organelles and cytosolic macromolecules to form an autophagosome, with the engulfed materials being delivered to the lysosome for degradation [69]. Briefly, autophagy proceeds through the capture of portions of cytoplasm containing target material inside expanding membranes, which finally enclose to form double-membrane vesicles called autophagosomes. Fully formed autophagosomes are shuttled along microtubules to lysosomes, whereupon fusion and degradation occur [70]. This removal and recycling serves as an emergency energy supply during starvation, but autophagy has also been linked to a diverse range of other protective roles [71, 72]. However, despite these pro-survival roles, autophagy has also been implicated as a mechanism of programmed cell death [73, 74]. Numerous studies have reported instances of dying cells displaying accumulated autophagosomes, which engulf large portions of the cell’s cytoplasm and which have been presumed to lead to excessive destruction of vital components [75, 76]. “Autophagic cell death” is morphologically defined as a type of cell death (type II) that occurs in the absence of chromatin condensation but accompanied by massive autophagic vacuolization of the cytoplasm [77].
4.1 Possible Autophagy Signaling Pathways in Cerebral Ischemia
The existence of autophagy in ischemic stroke has been found for many years; however, it is not sure whether autophagy plays a protective role in ischemic cerebral injury or not yet [78, 79]. Generally, in the neuronal system, moderate autophagy is thought to be neuroprotective because autophagy helps to clear aggregated-protein associated with neurodegeneration. Inadequate or defective autophagy may lead to neuronal cell death, while excess autophagy, often triggered by intensive stress, can also promote neuronal cell death.
Almost any signal can be a trigger for autophagy, some activating the pathway and some suppressing the pathway. By far, energy depletion and oxygen deficient environment are the most powerful triggers for stimulating autophagy, while the reverse environment factors, hormones, receptors with cytokine activities, receptors with tyrosine kinase activities and receptors that recognize pathogen ligands can also activate autophagy. Cerebral ischemia can activate multiple signaling pathways that subsequently feed into the autophagy pathway (Fig. 5.3).
The figure shows the many different signaling pathways involved in the activation of autophagy during cerebral ischemia. When activated, Akt and NF-κB activate mTOR to inhibit autophagy in cerebral ischemia. However, the activation of AMPK could inhibit the activity of mTOR and induce autophagy. Hypoxia caused by cerebral ischemia activates HIF-1α and induces autophagy through BNIP3 and p53. Excitotoxicity could induce autophagy by ER stress and block autophagic flux by glutamate in cerebral ischemia. Autophagy could also be induced through ROS and inhibited through PPAR-γ. PPAR-γ: Peroxisome proliferator-activated receptor-γ; AMP: Adenosine 5′-monophosphate; PI3K: phosphatidylinositol 3-kinase; ROS: reactive oxygen species; HIF-1α: hypoxia inducible factor 1α; Bcl-2: B cell lymphoma/leukmia-2; Bcl-xL: B-cell lymphoma-extra large; AMPK: AMP-activated protein kinase; AMPK: AMP-activated protein kinase; Akt/PKB: protein kinase B; NF-κB: nuclear factor kappa B; ER: endoplasmic reticulum; BNIP3: Bcl-2 and adenovirus E1B 19 kDa interacting proteins 3; mTOR: mammalian target of rapamycin.
-
1.
PI3K-Akt-mTORC1 mTOR is a 289 kDa serine/threonine protein kinase that regulates transcription, cytoskeleton organization, cell growth and cell survival. The mTOR is a high energy sensor, which on the other hand is a negative regulator of autophagy. By binding to different co-factors, mTOR can form two distinct protein complexes, mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2) [80]. mTORC1 is responsible for the inhibitory effect of rapamycin, more so than mTORC2. Recent studies suggest that the PI3K/Akt/mTOR pathway could regulate acute nervous system injury in cerebral hypoxia-ischemia [81]. PI3K consists of class I, class II and class III. Class I PI3K plays an important role in the PI3K-Akt-mTOR pathway. PI3K phosphorylates and activates Akt which in turn phosphorylates and inactivates tuberous sclerosis complex (TSC) 1/2. Inactivated TSC1/2 increases the activation of Rheb which is part of the Ras family GTP-binding protein, and mTOR is subsequently activated. Autophagy is inhibited by activating mTOR [82]. Beclin-1, a component of the class III PI3K, is essential for the initial steps of autophagy and could also induce autophagy via the interaction with other components of the class III PI3K pathway in cerebral ischemia [83]. Peroxisome prolif-erator-activated receptor-γ (PPAR-γ), a member of nuclear hormone receptor superfamily, is a ligand-activated transcription factor. PPAR-γ activation antagonizes beclin-1-mediated autophagy via upregulation of Bcl-2/Bcl-xl which interact with beclin-1 in cerebral ischemia/reperfusion [84].
-
2.
AMPK-mTORC1 AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase and consists of three subunits: a catalytic α-subunit and regulatory β and γ-subunits. Each subunit appears to have distinct functions. The most studied is the catalytic α-subunit which contains a threonine phosphorylation site that when phosphorylated, activates AMPK. The status of nutrient and energy depletion is sensed and modulated by kinase B1 (LKB1), Ca2+/calmodulin-dependent kinase kinase beta (CaMKKβ) and transforming growth factor β activated kinase-1 (TAK1), resulting in phosphorylation of threonine residue at 172 position and activation of AMPK [85] and AMPK activation could subsequently inhibit the activity of mTOR to induce autophagy [86, 87].
-
3.
Beclin 1-Bcl-2 complex.
Beclin 1 was identified as a Bcl-2-interacting protein through its BH3 domain [88]. The binding of Bcl-2 to Beclin 1 disrupts the association of Beclin 1 with PI3K, hVps34 and p150, therefore inhibiting autophagy [89]. Intriguingly, only ER-localized, but not mitochondria-localized, Bcl-2 inhibits autophagy [89]. Under stress conditions, Beclin 1 is released and induces autophagy [90, 91]. As previously demonstrated, the expression of Beclin 1 in neurons is dramatically increased in neonatal HI or focal cerebral ischemia [92, 93]. Ischemia stimulates autophagy through the AMPK–mTOR pathway, whereas ischemia/reperfusion stimulates autophagy through a Beclin 1-dependent but AMPK-independent pathway [94]. Although there are several different mechanisms to regulate the dissociation of Beclin 1 from Bcl-2 during autophagy in mammalian cells [95], the specific mechanism in cerebral ischemia is not yet established.
Hypoxia-inducible factor 1 (HIF-1) is a key transcriptional factor that is activated in response to hypoxia during cerebral ischemia [96]. HIF-1 is composed of a constitutively expressed HIF-1β subunit and an inducibly expressed HIF-1α subunit. Since ubiquitination is inhibited under hypoxic conditions, HIF-1α can accumulate and dimerize with HIF-1β. This dimer activates transcription of a number of downstream hypoxia-responsive genes, including vascular endothelial growth factor (VEGF), erythropoietin (EPO), glucose transporter 1, and glycolytic enzymes [97]. Bcl-2 and adenovirus E1B 19 kDa interacting proteins 3 (BNIP3) with a single Bcl-2 homology 3 (BH3) domain is a subfamily of Bcl-2 family proteins and also serves as an important target gene of HIF-1α [98]. BNIP3 can compete with beclin-1 for binding to Bcl-2 and beclin-1 is released to trigger autophagy [99]. BNIP3 also binds and inhibits Rheb, an upstream activator of mTOR, so it could activate autophagy by inhibiting mTOR activity. The induced p53 stabilization by up-regulation of HIF-1α also plays an important role in post-ischemic autophagy activation [97].
-
4.
p53 The tumor suppressor and transcription factor p53 has been reported to be pivotal in neuronal apoptosis [100]. Crighton et al. demonstrated that p53 induced autophagy through the upregulation of damage-regulated autophagy modulator (DRAM), the p53 target gene encoding a lysosomal protein [101].Other study also demonstrated that the NF-κB-regulated p53 pathway contributes to excitotoxic neuronal death by activating the autophagic process [102]. Overstimulation of N-methyl-d-aspartate receptors (NMDARs) induces the upregulation of p53, its target gene DRAM, and other autophagic proteins including LC3 and Beclin 1. Moreover, the NF-κB inhibitor SN50 inhibits the excitotoxin-induced upregulation of p53, its target gene DRAM, and other autophagic proteins.
Nuclear factor kappa B (NF-κB) is a transcription factor that regulates expression of multiple genes [103]. Recent experiments have demonstrated that the knockout of p50 (NF-κB1) enhanced autophagy by repression of mTOR in cerebral ischemic mice [104]. NF-κB-dependent p53 signal transduction pathway is also associated with autophagy and apoptosis in the rat hippocampus after cerebral ischemia/reperfusion insult [105]. Mitogen-activated protein kinases (MAPKs) include extracellular signal-related kinase (ERK), Jun NH2 terminal kinase (JNK) and p38 [106]. MAPK is one upstream regulator of mTORC1 and autophagy could also be induced via MAPK-mTOR signaling pathway in cerebral ischemia/reperfusion [107].
-
5.
Others.
Autophagic cell death is activated in the nervous system in response to oxidative stress [108]. Oxidative stress can occur in cerebral ischemia and could increase reactive oxygen species such as superoxide, hydroxyl radical and hydrogen peroxide. Recent studies have reported that selenium provides neuroprotection through preserving mitochondrial function, decreasing reactive oxygen species production and reducing autophagy [109]. Autophagy can also be induced under conditions of excitotoxicity which can also occur in cerebral ischemia [110]. Although excitotoxic glutamate blocks autophagic flux, it could also induce autophagy in hippocampal neurons [111]. Sustained elevations of Ca2+ in the mitochondrial matrix are a major feature of the intracellular cascade of lethal events during cerebral ischemia. Recently, it was reported that endoplasmic reticulum stress is one of the effects of excitotoxicity [1]. When endoplasmic reticula were exposed to toxic levels of excitatory neurotransmitters, Ca2+ was released via the activation of both ryanodine receptors and IP3R, leading to mitochondrial Ca2+ overload and activation of apoptosis. During endoplasmic reticulum stress, Ca2+ increase seems to be required for activating autophagy.
4.2 The Dual Roles of Autophagy in Cerebral Ischemia
Numerous data have demonstrated that autophagy is activated by ischemic insult in various models, and the elevated autophagic activity could be regulated by a wide range of interventions, mainly including pharmacological and genetic methods. There is no question that disrupting the autophagic process in brain is deleterious, particularly for the lifespan of the animal, resulting in the accumulation of dysfunctional or aging macromolecules and organelles [112, 113]. However, upon the acute cerebral ischemia stress, whether autophagy plays a beneficial or harmful role in the survival of neuronal cells is not an easy question. Adhami et al. [114] showed for the first time that many damaged neurons displayed features of autophagic/lysosomal cell death, and very few cells completed the apoptosis process in cerebral ischemic stress. This result suggested that the damaged neuronal cells can exhibit multiple forms of cell death morphological features, and autophagy is only one kind of cell death during ischemic injury. Alternatively, autophagy may protect neurons by degrading damaged organelles to abrogate apoptosis or generating energy to delay the onset of ionic imbalance and necrosis after cerebral ischemia–hypoxia. However, these early reports did not determine the exact role of autophagy. Dozens of later investigations pointed out the complex effects of autophagy in cerebral ischemia. The autophagy and the controversial impacts of autophagy on cerebral ischemic injury as a double-edged sword have been uncovered.
4.2.1 Detrimental Role of Autophagy in Ischemic Cerebral Injury
Mice deficient in Atg7, the gene essential for autophagy induction, showed nearly complete protection from both hypoxia-ischemia-induced caspase-3 activation and neuronal death, indicating autophagy is essential in triggering neuronal death after hypoxia-ischemia injury [115]. Wen et al. [116] confirmed autophagy was activated in a permanent middle cerebral artery occlusion (MCAO) model. In their paper, the infarct volume, brain edema and motor deficits could be significantly reduced by administration of 3-MA (an autophagy inhibitor). The neuroprotective effects of 3-MA were associated with an inhibition of ischemia-induced upregulation of LC3-II, a marker of active autophagosomes and autophagolysosomes. Moreover, it was observed that the inhibition of autophagy, either by direct inhibitor 3-MA or by indirect inhibitor 2ME2 (an inhibitor of hypoxia inducible factor-1α; HIF-1α) might prevent pyramidal neuron death after ischemia [97].
4.2.2 Beneficial Role of Autophagy in Cerebral Ischemic Injury
Carloni et al. [117] suggested that in neonatal hypoxia-ischemia, autophagy may be part of an integrated pro-survival signaling complex that includes PI3K-Akt-mTOR. When either autophagy or PI3K-Akt-mTOR pathways were interrupted, cells underwent necrotic cell death. Wang et al. [118] reported that neuronal survival was promoted during cerebral ischemia when autophagy was induced by nicotinamide phosphoribosyltransferase (Nampt, also known as visfatin), which is the rate-limiting enzyme in mammalian NAD+ biosynthesis and regulates the TSC2-mTOR-S6K1 signaling pathway. These studies suggest that autophagy may be a potential target for post-ischemic neuronal protection.
4.3 The Factors Determining the Role of the Autophagy in Cerebral Ischemia
4.3.1 The Degree of Autophagy Determines the Fate of Cells in Cerebral Ischemia
Kang and Avery [119] proposed that levels of autophagy were critical for the survival or death of cells: physiological levels of autophagy promote survival, whereas insufficient or excessive levels of autophagy promote death. This hypothesis was confirmed in an oxygen and glucose deprivation model that observed dual roles of the autophagy inhibitor 3-MA in different stages of re-oxygenation [75]. Twenty-four hours prior to reperfusion, 3-MA triggered a high rate of neuronal death. However, during 48–72 h of reperfusion, 3-MA significantly protected neurons from death. It is possible that prolonged oxygen and glucose deprivation/reperfusion triggers excessive autophagy, switching its role from protection to deterioration.
4.3.2 The Time at Which Autophagy Is Induced Determines Its Role
Autophagy could play a protective role in ischemic preconditioning but have a different effect once ischemia/reperfusion has occurred [120]. Infarct volume, brain edema and motor deficits induced by permanent focal ischemia were significantly reduced after ischemic preconditioning treatment. 3-MA suppressed neuroprotection induced by ischemic preconditioning, while rapamycin reduced infarct volume, brain edema and motor deficits induced by permanent focal ischemia [121]. This hypothesis was supported by a study by Yan et al. [122] in which 3-MA administrated through intracere-broventricular injection before hyperbaric oxygen preconditioning, attenuated the neuroprotection of hyperbaric oxygen preconditioning against cerebral ischemia. Moreover, 3-MA treatment before middle cerebral artery occlusion aggravated subsequent cerebral ischemic injury. In contrast, Carloni et al. [92, 117] found that when 3-MA and rapamycin were injected 20 min before hypoxia-ischemia, 3-MA inhibited autophagy, significantly reduced beclin-1 expression and caused neuronal death, while rapamycin increased autophagy and decreased brain injury. In addition, 3-MA administrated by intracerebroventricular injections strongly reduced the lesion volume (by 46%) even when given 4 h after the beginning of the ischemia [123]. Gao et al. [124] found that rapamycin applied at the onset of reperfusion might attenuate the neuroprotective effects of ischemic postconditioning. Conversely, 3-MA administered before reperfusion significantly reduced infarct size and abolished the increase of brain water content after ischemia. Targeting autophagy either pre- or post-treatment has different results and this may reflect the different effects of autophagy at early and late stages. The time of intervention could be related to the degree of autophagy at different stages of ischemia and further studies are necessary to confirm this.
4.3.3 Autophagy May Be Interrupted in Cerebral Ischemia
A common feature of many neurodegenerative diseases is the accumulation of an abnormally large number of autophagic vacuoles (autophagosomes and autolysosomes) or the frequent appearance of irregularly shaped autophagic vacuoles. Enhanced autophagosome formation seems to be reflected by increased density of autophagic vacuoles, but these increased autophagic vacuoles may also imply impaired autolysosomal degradation [125]. Rami et al. [93] also observed a dramatic up-regulation of Beclin-1 and LC3 in rats after cerebral ischemia. These results indicate that autophagy was activated in the brain following ischemia. Recently, however, it has been hypothesized that the increase in proteins may reflect a failure in lysosomal function leading to an accumulation of autophagosomes, or an improvement in the activity of autophagy [126]. Other studies found that accumulation of LC3-II was observed in sham-operated rats after treatment with lysosomal inhibitor-chloriquine, but the further change of LC3-II levels in post-ischemic brain tissues was not observed [127, 128]. The results indicated that accumulation of autophagy-associated protein following ischemia could be the result of failure of the autophagy pathway. Puyal and Clarke [129] found that lysosomal activity detected by LAMP-1 and cathepsin D was increased in neurons with punctate LC3 expression in neonatal focal cerebral ischemia model. The failure of autophagosome and lysosome fusion caused an increase of autophagosomes. The deficiency of acid phosphatase activity in the lysosome could lead to the increase of autophagosomes and autolysosomes. Further studies are required to verify whether the activity of autophagy is enhanced in cerebral ischemia.
5 Crosstalk Between Apoptosis, Autophagy and Necroptosis (Necrosis) After CIRI
PCD in vivo involves the complex interaction between apoptosis, autophagy, and necroptosis [130, 131] (Fig. 5.4). In some cases, a specific stimulus triggers only one type of programmed cell death, but in other situations, the same stimulus may initiate multiple cell death processes. Different types of mechanisms may co-exist and interact with each other within a cell, but ultimately, one mechanism dominates the others. The decision taken by a cell to undergo apoptosis, autophagy, or necroptosis is regulated by various factors, including the energy/ATP levels, the extent of damage or stress, and the presence of inhibitors of specific pathways (e.g., caspase inhibitors). ATP depletion activates autophagy. However, if autophagy fails to maintain the energy levels, necroptosis occurs [132]. Slight/moderate damage and low levels of death signaling typically induce apoptosis, whereas severe damage and high levels of the death signaling often result in necroptosis. Similarly, inhibition of caspase activity might change apoptosis to necrosis or autophagic cell death, whereas activation of calpain-mediated cleavage of autophagy-regulated protein, Atg-5, switches the mode of cell death from autophagy to apoptosis [133, 134]. Interestingly, although necroptosis, necrosis and secondary necrosis following apoptosis, represent different modes of cell death, all of them might eventually converge on similar cellular disintegration features, albeit with different kinetics [135]. Furthermore, apoptosis and autophagy differ from the necrosis by the feature of tissue inflammation [136]. Both apoptosis and autophagy do not exhibit tissue inflammation, while the latter does. Thus, learning more about the molecular mechanisms regulating various cell death modalities and their cross-talk is very important, since they play a critical role in CIRI.
Although death-receptor mediated apoptosis represents a canonical apoptotic pathway, stimulation of death receptors under apoptotic deficient conditions is now known to activate necroptosis [58]. The activation of death receptors by their respective ligands, such as FasL (CD95L) and TNF-α, respectively, leads to the formation of DISC (death-inducing signaling complex) that includes the adaptor protein FADD (Fasassociated death domain), caspase-8 and death domain-containing kinase RIP1. In apoptotic proficient condition, the recruitment of caspase-8 leads to its activation which in turn activates downstream caspases, such as caspase-3, and mitochondrial damage by cleaving Bid [137]. In apoptotic deficient cells when caspases cannot be activated, however, stimulation of death receptors leads to the activation of RIP1 kinase and necroptosis [54, 65]. Activation of AKT also appears to act as a switch, in addition to facilitating the necroptotic response, it also acts to inhibit apoptosis [138, 139]. These results clearly illustrate that the molecular pathways regulating death ligand-induced apoptosis and necroptossis are intimately intertwined. They also firmly establish the paradigm that inhibition of caspase-dependent apoptosis primes cells towards necroptosis.
Crosstalk between apoptosis and autophagy in CIRI is also complex. It has been acknowledged that appropriately controlled autophagy can induce neuroprotection and can rescue neurons from apoptotic cell death in the cerebral ischemia. For instance, clearance of damaged mitochondria via autophagy prevented neurons from caspase-dependent apoptosis [140]. However, autophagy may also act as a pro-apoptotic mechanism [99] and is causally connected with the subsequent onset of apoptotic cell death [141,142,143]. Cathepsin B, a protease which is normally confined inside the lysosomal-endosomal compartment, leaked from the lysosomes into the cytoplasm, initiating and promoting the execution of apoptosis [141]. It has been hypothesized that when the autophagic flux impairs, autolysosomes would extensively accumulate and the autophagic stress would be induced [126]. This would lead to autolysosomes and lysosomes membrane destabilization, which results in leakage of hydrolases, and subsequently provoke apoptosis [144]. As a result, the initial autophagy, as a defensive reaction, when over-activated, is converted into a damage response [75]. In this case, the inhibition of autophagy attenuates apoptotic cascades in ischemic injury. These evidences demonstrated that elucidating the interrelationships between autophagy and apoptosis will present novel opportunities for discovering targets in the therapy for cerebral ischemic injury.
So far, with the identification of several key molecules (e.g., ATG, Bcl-2 family members, Beclin 1, and p53) [105, 145,146,147,148,149,150], the mechanisms underlying the autophagy-apoptosis conversation are beginning to be uncovered. However, current researches seemingly only reveal a tip of the iceberg among the intricate interactions between autophagic and apoptotic cascades during the cerebral ischemic injury. Thus, more studies about the crosstalk between autophagy and apoptosis are warranted in the future.
The autophagy and necrosis can be activated in parallel or sequentially, and have either common or opposite objectives. The molecular underpinnings of this relationship remain largely elusive and somewhat controversial; autophagy has been shown to either promote [123] or suppress necroptosis (necrosis) [92, 151]. However, the ability of autophagy to suppress various forms of necrotic cell death is considered to be one of the most important pro-survival functions of autophagy that is achieved either by blocking apoptosis or suppressing necrotic cell death.
Therefore, because of the complex crosstalk between cell death pathways, much effort should be put on the finding of biomarkers that may predict the risk of a hypoxic-ischemic condition during the CIRI to initiate the treatment in an early stage, allowing the possibility of using the preconditioning effect of putative drugs. These early treatments may be followed by endovascular recanalization therapy (thrombolytic therapy or arterial embolectomy), that potentially reduces both apoptosis and necrosis. Of course, a better understanding of the mechanisms responsible for the switch among the different cell death phenotypes and the development of new and more selective molecules that can act upstream of these putative checkpoints will help to find new pharmacological strategies that could be associated to endovascular recanalization therapy.
References
Ouyang YB, Giffard RG. Er-mitochondria crosstalk during cerebral ischemia: Molecular chaperones and er-mitochondrial calcium transfer. Int J Cell Biol. 2012;2012:493934.
Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568.
Baron JC. Mapping the ischaemic penumbra with pet: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201.
Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke. 1981;12:723–5.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–20.
Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6.
Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18.
Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–66.
Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–9.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–9.
Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–76.
Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–27.
Rai NK, Tripathi K, Sharma D, Shukla VK. Apoptosis: a basic physiologic process in wound healing. Int J Low Extrem Wounds. 2005;4:138–44.
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–68.
Siegel C, McCullough LD. Nad+ depletion or par polymer formation: which plays the role of executioner in ischaemic cell death? Acta Physiol. 2011;203:225–34.
Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampaio N, Morin D, et al. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci. 2001;21:7127–34.
Hardwick JM, Chen YB, Jonas EA. Multipolar functions of bcl-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012;22:318–28.
Webster KA, Graham RM, Thompson JW, Spiga MG, Frazier DP, Wilson A, et al. Redox stress and the contributions of bh3-only proteins to infarction. Antioxid Redox Signal. 2006;8:1667–76.
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective bcl-2 family inhibitors. Nat Rev Drug Discov. 2017;16:273–84.
Zhai D, Chin K, Wang M, Liu F. Disruption of the nuclear p53-gapdh complex protects against ischemia-induced neuronal damage. Mol Brain. 2014;7:20.
Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–37.
Wang Y, Wan C, Yu S, Yang L, Li B, Lu T, et al. Upregulated expression of nf-yc contributes to neuronal apoptosis via proapoptotic protein bim in rats' brain hippocampus following middle cerebral artery occlusion (mcao). J Mol Neurosci. 2014;52:552–65.
Armugam A, Cher CD, Lim K, Koh DC, Howells DW, Jeyaseelan K. A secretory phospholipase a2-mediated neuroprotection and anti-apoptosis. BMC Neurosci. 2009;10:120.
Li D, Li X, Wu J, Li J, Zhang L, Xiong T, et al. Involvement of the jnk/foxo3a/bim pathway in neuronal apoptosis after hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2015;10:e0132998.
Ma M, Wang X, Ding X, Teng J, Shao F, Zhang J. Numb/notch signaling plays an important role in cerebral ischemia-induced apoptosis. Neurochem Res. 2013;38:254–61.
Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–67.
Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007;1772:947–57.
Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, et al. Apoptosis-inducing factor triggered by poly(adp-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–72.
Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, et al. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25.
Cho BB, Toledo-Pereyra LH. Caspase-independent programmed cell death following ischemic stroke. J Investig Surg. 2008;21:141–7.
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, et al. Mitochondrio-nuclear translocation of aif in apoptosis and necrosis. FASEB J. 2000;14:729–39.
Love S. Apoptosis and brain ischaemia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:267–82.
Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, et al. Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci. 2004;24:2750–9.
Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, et al. Cloning of a novel apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24:6189–201.
Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, et al. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome c and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–22.
Gao Y, Liang W, Hu X, Zhang W, Stetler RA, Vosler P, et al. Neuroprotection against hypoxic-ischemic brain injury by inhibiting the apoptotic protease activating factor-1 pathway. Stroke. 2010;41:166–72.
Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9.
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J, et al. Cd95 ligand (fas-l/apo-1l) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–17.
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by fty720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–74.
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85:396–402.
Chen S, Peng H, Rowat A, Gao F, Zhang Z, Wang P, et al. The effect of concentration and duration of normobaric oxygen in reducing caspase-3 and -9 expression in a rat-model of focal cerebral ischaemia. Brain Res. 2015;1618:205–11.
Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–44.
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, et al. Inhibition of caspases increases the sensitivity of l929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85.
Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by fas-associated protein with death domain. J Cell Biol. 1998;143:1353–60.
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14.
Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, et al. Caspase-8 regulates tnf-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9.
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-flip(l) complex inhibits ripk3-dependent necrosis. Nature. 2011;471:363–7.
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. Rip3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72.
Xu Y, Wang J, Song X, Qu L, Wei R, He F, et al. Rip3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via aif. Sci Rep. 2016;6:29362.
Zhang J, Yang Y, He W, Sun L. Necrosome core machinery: Mlkl. Cell Mol Life Sci. 2016;73:2153–63.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase rip as effector molecule. Nat Immunol. 2000;1:489–95.
Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, et al. The rip1/rip3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–50.
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of rip3 kinase. Cell. 2012;148:213–27.
Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase pgam5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–43.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9.
Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, et al. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous rip3. Neurobiol Dis. 2014;68:26–36.
Yin B, Xu Y, Wei RL, He F, Luo BY, Wang JY. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury. Brain Res. 2015;1609:63–71.
Fakharnia F, Khodagholi F, Dargahi L, Ahmadiani A. Prevention of cyclophilin d-mediated mptp opening using cyclosporine-a alleviates the elevation of necroptosis, autophagy and apoptosis-related markers following global cerebral ischemia-reperfusion. J Mol Neurosci. 2017;61:52–60.
Dong Y, Bao C, Yu J, Liu X. Receptor-interacting protein kinase 3-mediated programmed cell necrosis in rats subjected to focal cerebral ischemia-reperfusion injury. Mol Med Rep. 2016;14:728–36.
Miao W, Qu Z, Shi K, Zhang D, Zong Y, Zhang G, et al. Rip3 s-nitrosylation contributes to cerebral ischemic neuronal injury. Brain Res. 2015;1627:165–76.
Melo-Lima S, Celeste Lopes M, Mollinedo F. Necroptosis is associated with low procaspase-8 and active ripk1 and −3 in human glioma cells. Oncoscience. 2014;1:649–64.
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of rip1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21.
Vandenabeele P, Declercq W, Vanden Berghe T. Necrotic cell death and 'necrostatins': Now we can control cellular explosion. Trends Biochem Sci. 2008;33:352–5.
Li H, Gao A, Feng D, Wang Y, Zhang L, Cui Y, et al. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Transl Stroke Res. 2014;5:618–26.
Xu Y, Wang J, Song X, Wei R, He F, Peng G, et al. Protective mechanisms of ca074-me (other than cathepsin-b inhibition) against programmed necrosis induced by global cerebral ischemia/reperfusion injury in rats. Brain Res Bull. 2016;120:97–105.
Yoshimori T. Autophagy: paying Charon’s toll. Cell. 2007;128:833–6.
Shibutani ST, Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res. 2014;24:58–68.
Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–8.
Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–57.
Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16:21–30.
Clarke PG, Puyal J. Autophagic cell death exists. Autophagy. 2012;8:867–9.
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18:250–60.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an atg7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2.
Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis. 2011;43:52–9.
Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3:1124–6.
Jung CH, Ro SH, Cao J, Otto NM, Kim DH. Mtor regulation of autophagy. FEBS Lett. 2010;584:1287–95.
Chong ZZ, Shang YC, Wang S, Maiese K. A critical kinase cascade in neurological disorders: Pi 3-k, akt, and mtor. Future Neurol. 2012;7:733–48.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12.
Xingyong C, Xicui S, Huanxing S, Jingsong O, Yi H, Xu Z, et al. Upregulation of myeloid cell leukemia-1 potentially modulates beclin-1-dependent autophagy in ischemic stroke in rats. BMC Neurosci. 2013;14:56.
Xu F, Li J, Ni W, Shen YW, Zhang XP. Peroxisome proliferator-activated receptor-gamma agonist 15d-prostaglandin j2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS One. 2013;8:e55080.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. Tsc2 integrates wnt and energy signals via a coordinated phosphorylation by ampk and gsk3 to regulate cell growth. Cell. 2006;126:955–68.
Poels J, Spasic MR, Callaerts P, Norga KK. Expanding roles for amp-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009;31:944–52.
Li J, McCullough LD. Effects of amp-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–92.
Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the bcl-xl-beclin 1 peptide complex: Beclin 1 is a novel bh3-only protein. J Biol Chem. 2007;282:13123–32.
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell. 2005;122:927–39.
He C, Levine B. The beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–9.
Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the beclin 1 interactome. EMBO J. 2010;29:515–6.
Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–39.
Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–41.
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of amp-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22.
Kang R, Zeh HJ, Lotze MT, Tang D. The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–80.
Althaus J, Bernaudin M, Petit E, Toutain J, Touzani O, Rami A. Expression of the gene encoding the pro-apoptotic bnip3 protein and stimulation of hypoxia-inducible factor-1alpha (hif-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int. 2006;48:687–95.
Xin XY, Pan J, Wang XQ, Ma JF, Ding JQ, Yang GY, et al. 2-Methoxyestradiol attenuates autophagy activation after global ischemia. J Can Sci Neurol. 2011;38:631–8.
Cho B, Choi SY, Park OH, Sun W, Geum D. Differential expression of bnip family members of bh3-only proteins during the development and after axotomy in the rat. Mol Cells. 2012;33:605–10.
He S, Wang C, Dong H, Xia F, Zhou H, Jiang X, et al. Immune-related gtpase m (irgm1) regulates neuronal autophagy in a mouse model of stroke. Autophagy. 2012;8:1621–7.
Banasiak KJ, Haddad GG. Hypoxia-induced apoptosis: Effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 1998;797:295–304.
Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, et al. Dram, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–34.
Wang Y, Dong XX, Cao Y, Liang ZQ, Han R, Wu JC, et al. P53 induction contributes to excitotoxic neuronal death in rat striatum through apoptotic and autophagic mechanisms. Eur J Neurosci. 2009;30:2258–70.
Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, et al. Low-level laser therapy activates nf-kb via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6:e22453.
Li WL, Yu SP, Chen D, Yu SS, Jiang YJ, Genetta T, et al. The regulatory role of nf-kappab in autophagy-like cell death after focal cerebral ischemia in mice. Neuroscience. 2013;244:16–30.
Cui DR, Wang L, Jiang W, Qi AH, Zhou QH, Zhang XL. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the nf-kappab/p53 signaling pathway. Neuroscience. 2013;246:117–32.
Lien SC, Chang SF, Lee PL, Wei SY, Chang MD, Chang JY, et al. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, smad1/5, and p38 mapk. Biochim Biophys Acta. 2013;1833:3124–33.
Wang PR, Wang JS, Zhang C, Song XF, Tian N, Kong LY. Huang-lian-jie-du-decotion induced protective autophagy against the injury of cerebral ischemia/reperfusion via mapk-mtor signaling pathway. J Ethnopharmacol. 2013;149:270–80.
Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, et al. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem. 2010;285:667–74.
Mehta SL, Kumari S, Mendelev N, Li PA. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012;13:79.
Puyal J, Ginet V, Grishchuk Y, Truttmann AC, Clarke PG. Neuronal autophagy as a mediator of life and death: contrasting roles in chronic neurodegenerative and acute neural disorders. Neuroscientist. 2012;18:224–36.
Kulbe JR, Mulcahy Levy JM, Coultrap SJ, Thorburn A, Bayer KU. Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res. 2014;1542:12–9.
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in atg7-deficient mice. J Cell Biol. 2005;169:425–34.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4.
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–83.
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–69.
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–9.
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and akt/creb signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–77.
Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87.
Kang C, Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82–4.
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–435.
Sheng R, Zhang LS, Han R, Liu XQ, Gao B, Qin ZH. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy. 2010;6:482–94.
Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011;1402:109–21.
Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–89.
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, et al. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One. 2012;7:e46092.
Komatsu M, Ueno T, Waguri S, Uchiyama Y, Kominami E, Tanaka K. Constitutive autophagy: vital role in clearance of unfavorable proteins in neurons. Cell Death Differ. 2007;14:887–94.
Xu F, Gu JH, Qin ZH. Neuronal autophagy in cerebral ischemia. Neurosci Bull. 2012;28:658–66.
Luo T, Park Y, Sun X, Liu C, Hu B. Protein misfolding, aggregation, and autophagy after brain ischemia. Transl Stroke Res. 2013;4:581–8.
Liu C, Gao Y, Barrett J, Hu B. Autophagy and protein aggregation after brain ischemia. J Neurochem. 2010;115:68–78.
Puyal J, Clarke PG. Targeting autophagy to prevent neonatal stroke damage. Autophagy. 2009;5:1060–1.
Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31:5045–60.
Zhivotovsky B, Orrenius S. Cell death mechanisms: cross-talk and role in disease. Exp Cell Res. 2010;316:1374–83.
Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–9.
Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–87.
Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G, et al. Fangchinoline induces autophagic cell death via p53/sestrin2/ampk signalling in human hepatocellular carcinoma cells. Br J Pharmacol. 2011;164:731–42.
Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17:922–30.
Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14:51–9.
Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–206.
Jeong SJ, Dasgupta A, Jung KJ, Um JH, Burke A, Park HU, et al. Pi3k/akt inhibition induces caspase-dependent apoptosis in htlv-1-transformed cells. Virology. 2008;370:264–72.
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. Pi3k/akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204.
Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9:1321–33.
Zhang ZB, Li ZG. Cathepsin b and phospo-jnk in relation to ongoing apoptosis after transient focal cerebral ischemia in the rat. Neurochem Res. 2012;37:948–57.
Canu N, Tufi R, Serafino AL, Amadoro G, Ciotti MT, Calissano P. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J Neurochem. 2005;92:1228–42.
Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–31.
Heitz S, Grant NJ, Leschiera R, Haeberle AM, Demais V, Bombarde G, et al. Autophagy and cell death of purkinje cells overexpressing doppel in ngsk prnp-deficient mice. Brain Pathol. 2010;20:119–32.
Radoshevich L, Murrow L, Chen N, Fernandez E, Roy S, Fung C, et al. Atg12 conjugation to atg3 regulates mitochondrial homeostasis and cell death. Cell. 2010;142:590–600.
He G, Xu W, Tong L, Li S, Su S, Tan X, et al. Gadd45b prevents autophagy and apoptosis against rat cerebral neuron oxygen-glucose deprivation/reperfusion injury. Apoptosis. 2016;21:390–403.
Qi Z, Dong W, Shi W, Wang R, Zhang C, Zhao Y, et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl Stroke Res. 2015;6:198–206.
Delgado M, Tesfaigzi Y. Bh3-only proteins, bmf and bim, in autophagy. Cell Cycle. 2013;12:3453–4.
Luo S, Rubinsztein DC. Apoptosis blocks beclin 1-dependent autophagosome synthesis: an effect rescued by bcl-xl. Cell Death Differ. 2010;17:268–77.
Luo S, Rubinsztein DC. Bcl2l11/bim: a novel molecular link between autophagy and apoptosis. Autophagy. 2013;9:104–5.
Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury: evidence and speculations. Autophagy. 2009;5:221–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Wei, R., Xu, Y., Zhang, J., Luo, B. (2018). Programmed Cell Death in CIRI. In: Jiang, W., Yu, W., Qu, Y., Shi, Z., Luo, B., Zhang, J. (eds) Cerebral Ischemic Reperfusion Injuries (CIRI). Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-90194-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-90194-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-90193-0
Online ISBN: 978-3-319-90194-7
eBook Packages: MedicineMedicine (R0)