Abstract
Fever with renal syndrome is currently endemic in Eurasia, where the main etiological agents are the Hantaan and Seoul viruses in Asia (China, South Korea, and the Far East of Russia), in addition to the Seoul, Puumala, and Dobrava viruses in Europe (central, northern, Alpine Massif, Balkans, and western Russia). Lethality rates are higher with Hantaan and Dobrava virus infections (5–10%) when compared to the Puumala and Seoul viruses (1%). With the expansion and geographical migration of the urban rodent (Rattus norvegicus) from the “Old World,” the Seoul virus was introduced into the Americas and is now considered a virus with a cosmopolitan distribution. On the American continent, the presence of the Seoul virus has been confirmed in Brazil, Argentina, and the United States. The hantavirus transmission to humans occurs by inhalation of aerosol-dispersed viral particles present in rodent droppings and saliva. This disease should be clinically differentiated from leptospirosis and other viral hemorrhagic fevers that occur in the same areas of occurrence of hantavirus infections. There is no treatment with antiviral drugs specific for hantavirus. Faced to a suspected hantavirus case, it should be communicated to the local health authorities and provide an eventually intensive care unit support.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The first cases of hantavirus infections were reported during the Korean War (1951–1953), where soldiers who came into contact with rodent-infested environments developed fever, myalgia, hemorrhagic manifestations, acute renal failure, and hemodynamic instability [1,2,3].
Twenty-five years after this event, an RNA virus of the genus Hantavirus, family Bunyaviridae, was isolated from wild rodents of the species Apodemus agrarius. Subsequently, this new agent was called “Hantaan” in reference to the Korean River, where the first cases occurred, and the rodents were captured [4].
Soon after that, studies showed that the Korean hemorrhagic fever was not an exclusive disease of that region and was likely to occur in countries such as Russia, China, and Scandinavia [1]. So, it was verified that the Korean hemorrhagic fever was just one of the variants of a group of diseases that manifested by hemorrhage and acute renal failure, which led the World Health Organization to denominate it hemorrhagic fever with renal syndrome (HFRS) [1]. HFRS is caused by the viral variants: Hantaan, Seoul, Dobrava, and Puumala [5, 6].
In Brazil, the presence of the Seoul virus has been reported since the 1980s [7], with the subsequent description of infections in humans [8] and virus circulation in urban rodent populations of the genus Rattus [9, 10].
The first record of human hantavirus infection in the Americas was carried out in the United States in the Four Corners region (New Mexico, Arizona, Colorado, and Utah) in May 1993, where an outbreak of an acute respiratory disease called hantavirus pulmonary syndrome (HPS) was reported [11, 12].
In later studies, related to the first cases in South America, an important cardiac involvement was described, which resulted in the change of the nomenclature to hantavirus cardiopulmonary syndrome (HCPS) [13].
In Brazil, the first cases of HCPS were reported in November of the same year from an outbreak that occurred in a rural area of the municipality of Juquitiba, state of São Paulo. The similar cases showed clinical pictures that included respiratory failure, and their serological and histopathological exams confirmed hantavirus infection, thus characterizing it as the first outbreak of HCPS in Brazil [14].
14.2 Natural History of Hantavirus Infection
The perpetuation of hantavirus infection in nature occurs by rodent interactions associated with food competition. At times of food deficit or overcrowding, fights are frequent, which results in contact with saliva or excreted material, thus maintaining the infections in enzootic cycles [15]. Recent studies have also identified other mammals – bats, marsupials, and shrews – carrying hantavirus infections. However, the participation of these animals in enzootic and epidemic cycles is not yet well established [16].
The hantavirus transmission to humans occurs by inhalation of aerosol-dispersed viral particles present in rodent droppings and saliva [17]. Rarely, contagion can also occur through the bite of infected animals, inoculation into the skin or mucous membranes with solution of continuity, or ingestion of water or food contaminated by the virus [18].
Despite the severity of HCPS, oligosymptomatic and even asymptomatic cases of human hantavirus infection are known to occur. These cases are confirmed due to the presence of hantavirus antibodies in the general population, detected in serological surveys, including in individuals with no epidemiological history [19].
The incubation period of the disease may vary from a few days to 2 months. The minimum period recorded was 3 days, and the maximum period recorded was 60 days. Most cases show the first signs of the disease around 2 weeks after the exposure [18]. The median duration of the disease from symptom onset to cure or death is 5 and 13 days, respectively [20].
14.3 Characterization of Hantaviruses
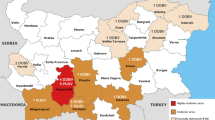
The viruses are grouped by strains that resemble each other according to their morphological, morphogenic, and antigenic properties [21]. Overall, hantavirus variants are named after their first detection and are divided into Old World and New World hantaviruses (Fig. 14.1) [21].
It is also known that different hantaviruses can produce more or less severe clinical pictures [21, 22]. Many of these viruses still have unknown pathogenicity to humans, with infections being described in rodents only [21, 23].
14.3.1 HFRS Epidemiological Situation
The HFRS is given different names throughout its distribution area: hemorrhagic nephritis in the former Soviet Union; songo fever or epidemic hemorrhagic fever in China; Korean hemorrhagic fever in Korea; epidemic nephropathy in Scandinavia; epidemic nephritis or epidemic hemorrhagic fever or Balkan nephritis in Europe; and epidemic hemorrhagic fever in Japan [24].
Based on the isolation and characterization of the Hantaan virus [25], it was verified that the HFRS had a geographical distribution across several Asian and European countries, such as Japan, China, Manchuria, and Russia, extending to other Southeast Asian countries and Africa [24, 26].
HFRS is currently endemic in Eurasia, where the main etiological agents are the Hantaan and Seoul viruses in Asia (China, South Korea, and the Far East of Russia), in addition to the Seoul, Puumala, and Dobrava viruses in Europe (central, northern, Alpine Massif, Balkans, and western Russia) [27]. Lethality rates are higher with Hantaan and Dobrava virus infections (5–10%) when compared to the Puumala and Seoul viruses (1%) [28] .
With the expansion and geographical migration of the rat (Rattus norvegicus) from the “Old World,” the Seoul virus was introduced into the Americas and is now considered a virus with a cosmopolitan distribution [29]. On the American continent, the presence of the Seoul virus has been confirmed in Brazil, Argentina, and the United States [7,8,9,10, 30,31,32].
14.3.2 Epidemiological Situation of HCPS
HCPS is distributed from Canada to southern Argentina. In North America, cases have already been identified in Canada, especially in the south of the country. In the United States, the cases have a fatality rate of around 40%–50%, being endemic in most of the American states, with clusters of cases in the American southwest [21, 26, 33].
There are no reported human cases of HCPS in Mexico, Central America, except Panama [34], and the Caribbean, although some hantaviruses have already been identified in rodents captured in Mexico and Costa Rica [35, 36].
In South America, besides Brazil, cases have already been confirmed in Argentina, Uruguay, Chile, Paraguay, Bolivia, Venezuela, and Colombia [18, 21, 35, 37,38,39,40,41].
In Argentina, in 1993, the first cases of HCPS outbreaks were reported and identified in people who had as a common risk situation the exposure to a place with agricultural production and grain planting, which attracts rodents. This country has three major transmission areas and has already characterized at least seven different viral genotypes. The average case fatality rate has been approximately 23% [42]. Also in Argentina, the first cases with proven person-to-person transmission were detected, caused by the Andes virus [43].
In Chile, HCPS was described in 1995, with most cases being reported in the south of the country. The reported cases were more frequent among males (72%), working in agricultural and/or forest environments (50%) and with a mean age of 31.5 years, with an overall case fatality rate of 40%–70% [38, 44].
Both in Argentina and Chile, where the main etiological agent of HCPS is the Andes virus, hemorrhagic manifestations and acute renal failure are common clinical characteristics in affected patients [45, 46].
In 1995, the first case of HCPS was recognized in Paraguay. The emergence of the disease, which occurs mainly in the Chaco Paraguayan region, was attributed to an invasion of rural homes by rats from a floodplain region that was flooded during torrential rain. The case fatality rate ranged from 10% to 20%, and approximately 38% of the patients were males with a mean age of 29 years. Hantavirus infection is quite common among the indigenous population, where prevalence coefficients of up to 40% have been detected [47].
In Uruguay, cases of HCPS have a case fatality rate of 25%, and since the first diagnosis in 1997, few cases have been reported. The disease most often affects young adult males (78.9%) and has been associated with rural activities or entering enclosed places or environments with the presence of rodents, being restricted to the southern region of the country [48].
Bolivia has few reports of cases, which sporadically occur since 1997 [49]. In 2002 and 2013, Venezuela and Colombia, respectively, reported their first cases [35, 41].
14.3.3 Epidemiological Situation of Hantaviruses in Brazil
HCPS is the main clinical pattern in Brazil. Cases have been identified in all regions (average of 150 cases per year), most frequently in the South, Southeast, and Midwest regions, with an average case fatality rate of 38.5% [40]. The highest incidences are detected in the States of Mato Grosso do Sul and Santa Catarina (Fig. 14.2), and there is no well-defined seasonality, with cases occurring throughout all months of the year, with certain regional variations [20]. The risk factors for hantavirus infections include involvement in agricultural, domestic, and leisure activities that are associated with human exposure to rodents or their excreted materials [20, 40]. Thus, men of economically productive age are the ones most often affected by HCPS.
In Brazil, six variants of hantavirus have been associated with HCPS: Juquitiba/Araucaria, Araraquara/Paranoá, Castelo dos Sonhos, Anajatuba, Laguna Negra, and Rio Mamoré [18, 21, 50, 51].
Although there are few current studies on the Seoul virus in Brazil [10], studies carried out in the late twentieth century indicate that the virus circulates in both humans and urban rodents of the genus Rattus, in addition to describing cases with clinically indistinguishable and HFRS-compatible manifestations, probably underdiagnosed in the presence of suspected cases of leptospirosis, of which clinical manifestations are similar [7,8,9,10].
14.4 HFRS Clinical Manifestations
HFRS is characterized by an incubation period of 7–42 days, during which subclinical or oligosymptomatic infections are not uncommon. Classically, clinical evolution is divided into five phases: febrile, hypotensive, oliguric, diuretic, and convalescence; these periods may overlap and, in mild cases, not even occur. The onset is abrupt and manifests with high fever, chills, retro-orbital headache, photophobia, myalgias, abdominal pain, nausea, and vomiting; diffuse cutaneous hyperemia affecting the face, neck, and upper chest, and petechiae on the soft palate and armpits are common physical findings. The liver can be palpated in a significant number of cases; liver impairment is more common in infections caused by the Seoul virus. Many patients recover slowly from this phase, but some develop hypotension and shock, which usually occur before the 5th or 6th day. The blood pressure drop may be mild, but some patients develop refractory shock, which requires the use of vasoactive drugs. Sudden albuminuria is frequent at the end of the fever phase. During the hypotensive phase, thrombocytopenia <70,000/mm3, proteinuria >3000 mg/day, decreased urinary density, hemoconcentration (hematocrit >50%), and leukocytosis are common, sometimes with leukemoid reaction.
Bleeding is common in the oliguric phase and can be observed in the conjunctiva, skin and mucosa, digestive tract, and central nervous system; microscopic hematuria is also frequent at this stage. Renal function deteriorates (increased creatinine and blood urea nitrogen), usually 24 hours after hypotension, with the onset of oliguria or even anuria, which requires the use of dialysis methods. Recovery from this moment onward can be rapid, with the onset of intense diuresis (above 3 L per day), hydroelectrolytic disorder (hypokalemia, hyponatremia, hyperphosphatemia), and episodes of arterial hypertension. Deaths are due to kidney failure in the oliguric phase and/or shock in the hypotensive, oliguric, or diuretic phase. This disease should be clinically differentiated from leptospirosis and other viral hemorrhagic fevers that occur in the same areas of occurrence of hantavirus infections [26, 52,53,54,55,56].
Although uncommon, some patients can have renal sequelae in the first months or years after the convalescence phase, such as increased glomerular filtration rate and proteinuria. However, such sequelae tend to disappear over time, without evidence of chronic kidney disease or end-stage renal failure [57].
Recent studies in Europe have indicated that smoking, in addition to being a common condition in patients infected with Puumala virus, is a risk factor for developing acute renal failure and severe disease [58].
14.5 Clinical Manifestations of HCPS
14.5.1 Prodromal Phase
In the prodromal phase, the most frequent manifestations are fever, myalgia, back pain, abdominal pain, asthenia, severe headache, and gastrointestinal symptoms, such as nausea, vomiting, and diarrhea (Fig. 14.3). This unspecific condition may last from 1 to 6 days or for up to 15 days, and then regress. Approximately 70% of the cases in Brazil develop into the cardiopulmonary clinical phase. Dry cough may already be present at the end of this stage [18].
14.5.2 Cardiopulmonary Phase
It is characterized by the onset of coughing, which is usually dry, although in some cases it may be productive, accompanied by tachycardia, tachydyspnea, and hypoxemia. Such manifestations may be followed by a rapid evolution to noncardiogenic pulmonary edema, hypotension, and circulatory collapse. The chest X-ray shows, in 60% of cases, bilateral diffuse interstitial infiltrate that rapidly evolves with alveolar filling, especially in the hila and pulmonary bases. Pleural effusion, mainly bilateral, of small magnitude, is a common finding. The cardiac area is normal. The cardiac index is low, and the peripheral vascular resistance is high, the opposite of what is observed in septic shock. Renal impairment may appear, but it is usually mild to moderate, although acute renal failure may occur, especially in infections by the Bayou, Black Creek Canal, and Andes virus. The case fatality rate is high at this stage, usually around 45% [17, 18].
14.6 Diagnosis
Laboratory diagnosis of cases of hantavirus human infection is commonly performed by the enzyme-linked immunosorbent assay (ELISA), which aims to detect mainly the IgM antibodies associated with recent infection. Such diagnosis is possible even in the acute phase of the disease, because antibodies in HCPS appear with the onset of signs and symptoms [13, 17, 18].
The reverse transcription polymerase chain reaction (RT-PCR) methodology , which detects hantavirus RNA in clinical samples, is extremely useful and practical for the diagnosis of HCPS [17, 18].
14.7 Laboratory Diagnosis
The laboratory tests, performed by reference laboratories for the Brazilian Ministry of Health, are as follows:
IgM-ELISA: Approximately 95% of HCPS patients have detectable IgM in a serum sample collected at symptom onset; thus, it is an effective method for the diagnosis of hantavirus infection.
Immunohistochemistry: Particularly, it is used for diagnosis in cases of death, when it was not possible to perform the serological diagnosis in vivo.
Reverse transcription-polymerase chain reaction (RT-PCR): It is useful for identifying the virus and its genotype, being considered a complementary test.
The IgG ELISA technique, although available in the public network, is used in epidemiological studies to detect previous viral infection in rodents or humans [13, 17, 18].
14.7.1 Nonspecific Laboratory Diagnosis
HCPS laboratory findings, although not characteristic, may support the diagnosis of a suspected case of the disease. Data from complete blood counts obtained during the prodromal period, such as elevated hematocrit, presence of immunoblasts (atypical lymphocytes), and thrombocytopenia, may be the evidence that respiratory impairment can occur within hours, even if the chest X-ray results are normal [59, 60]. The most commonly found chest X-ray alterations are progressive bilateral interstitial infiltrate, hilar and peribronchial congestion, and pleural effusion; after 24–48 hours, air-space consolidation rapidly evolves and pleural effusion progressively increases; if the evolution is favorable, these radiological abnormalities disappear within a few days [61].
Other signs and symptoms have been reported, and in some cases, the disease may not progress from the prodromal stage or clinical symptoms may be completely absent [59]. Based on clinical symptomatology, early disease recognition is not easy and may be mistaken by endemic diseases prevalent in the same areas, such as dengue, leptospirosis, and influenza [18, 26, 54, 62].
14.7.2 Differential Diagnosis
Diseases of infectious origin: leptospirosis, influenza and parainfluenza, dengue, Yellow fever and Rift Valley fever, Coxsackie virus infections, Adenovirus and Arenavirus (Lassa fever) infections, trichinellosis, malaria, pneumonia (viral, bacterial, fungal and atypical), septicemia, rickettsiosis, histoplasmosis, and pneumocystosis [18, 26, 54, 62].
Noninfectious diseases: acute abdomen of variable etiology, acute respiratory distress syndrome (ARDS), acute (cardiogenic) pulmonary edema, interstitial pneumonia by collagen disease (systemic lupus erythematosus, rheumatoid arthritis), and chronic obstructive bronchopulmonary disease (COBPD) [18, 26].
14.8 General Pathophysiology and Kidney Impairment
In both HFRS and HCPS, viral infections start with the endothelial cells of the lung microvascularization. After viral replication, the virus disseminates through the lymphatic route to other organs and tissues. The pathogenetic mechanisms of hantavirus infections seem to originate from an autoimmune response, since they do not induce increased capillary permeability by themselves. The disease severity increases after the immune response. The viral infection triggers an immune response, with the activation of defense cells, including thymus-dependent cytotoxic lymphocytes (L-TCD8) [55].
Recognized as an infectious vasculitis, small vessel endothelium is a major target in hantavirus infection, producing endothelial activation, vascular dysfunction secondary to the immune response and inflammatory mechanism, thrombin formation, fibrinolysis, and increased platelet consumption [63].
In addition to being massively present in the lungs, defense cells are also found in peripheral blood as atypical lymphocytes. Once activated, these cells are capable of producing cytokines that will act directly on the vascular endothelium, as well as stimulating macrophages to produce more cytokines, such as interleukin 1 (IL-1), interferon gamma (IFN-γ), and the tumor necrosis factor (TNF). These substances, acting directly on the capillary, can lead to increased vascular permeability, which allows massive fluid leakage into the interstitial space and later into the alveoli, triggering pulmonary edema and acute respiratory failure in the case of HCPS [55].
In the kidneys, especially in the podocytes, glomerular endothelial cells, and tubular epithelial cells, hantaviruses join and enter via αvβ1 integrins and components of the complement system (CD55 and GC1qR/p32) [64]. Under basal conditions, β-integrins contribute to the regulation of the vascular integrity, endothelial cell permeability, through restriction of vascular endothelial growth factor (VEGF), and in platelet functions [63]. The viral infection inhibits normal regulation of β-integrins, inducing an increased endothelial cell response to VEGF and producing a significant increase in vascular permeability [63]. Additionally, there is redistribution and decrease in intercellular junctions, explaining the classical proteinuria in the acute phase of the disease [63]. Urinary loss of low molecular weight proteins (α1 and β2 microglobulins) suggests that tubular involvement also contributes to proteinuria [63].
Increased vascular permeability in different organs, including the kidneys, explains the typical characteristics of hantavirus infection: hemoconcentration, hypotension, shock, abdominal pain (due to retroperitoneal edema), and pleural effusion [63].
To date, the reasons why there are groups of hantavirus that trigger greater pathogenesis in the renal system in the case of HFRS, or in the lungs and heart in the case of HCPS, are unknown [18].
14.9 General Considerations and Findings on Renal Biopsy
Hantavirus infections usually have little histopathological evidence of cell damage, and no pathognomonic lesions are found [62]. The lungs are the most frequently injured organs in HCPS, where pulmonary edema is described, with discrete hyaline membrane, interstitial lymphocyte infiltrate (immunoblasts), and activated macrophages [65, 66].
Renal hantavirus infection is typically described as acute tubulointerstitial nephritis [55]. Findings in the histopathological study include cell infiltrates (leukocytes, plasma cells, monocytes, macrophages, polymorphonuclear cells), edema and interstitial hemorrhages, medullary hemorrhages, alterations in the tubular epithelium and lumen, generalized capillary damage with intratubular alterations and interstitial edema, and sporadically, glomerular involvement with hypercellularity and mesangial expansion (Fig. 14.4) [55, 56, 58]. The severity of acute renal failure is due to the level of tubulointerstitial and glomerular damage [55]; however, glomerulonephritis is a rare consequence of hantavirus infection [56].
(a) Endothelial cells of glomerular capillaries containing hantavirus antigens detected by immunohistochemical technique. (b) Endothelial cells containing hantavirus antigens detected by immunohistochemical technique in the renal medullary capillaries. (a, b) Mouse monoclonal A1C5 for hantavirus, Abcam, alkaline phosphatase conjugated polymer, and fast red substrate, counterstained with hematoxylin; 40×. (Courtesy of Silvia D’Andretta Iglezias, Pathologist at the Instituto Adolfo Lutz, Pathological Anatomy Center, São Paulo)
A study published in 2015, carried out in France, characterized renal histopathological findings in 17 patients diagnosed with HFRS secondary to Puumala virus infection [67]. Interestingly, interstitial hemorrhage and acute tubular necrosis were commonly described, but interstitial nephritis was not frequent. Moreover, renal microvascular inflammation (presence of T cells and macrophages) was observed, with cortical peritubular capillaritis and in the medullary portion of the vasa recta.
14.10 Treatment
There is no treatment with antiviral drugs specific for hantavirus. Any suspected case of hantavirus should be transferred to the intensive care unit (ICU) as soon as possible [18].
Unspecific/prodromal form : The treatment of patients with mild forms of the disease is symptomatic. Hydration, when necessary, should be carefully provided to avoid volume overload. Strict control of vital data regarding hemodynamic and ventilatory parameters is required to prevent triggering or worsening of the cardiorespiratory condition in the case of HCPS [18].
Severe form: In patients with more severe forms and worsening hemodynamic and ventilatory parameters, careful intravenous (IV) fluid infusion is recommended, which, if excessive, may precipitate pulmonary edema. Adequate management of fluid intake is the main therapeutic element. Fluid balance is another parameter of great importance, requiring control of diuresis, bladder catheterization (not mandatory), and renal function. The volume of IV fluids should be sufficient to maintain the preload and ensure adequate renal plasma flow, maintaining a negative or at least zero fluid balance, so as not to increase pulmonary edema (maximum 2500 mL in 24 hours for adults). Colloidal and plasma solutions may be employed to achieve a negative or zero fluid balance, sufficient to optimize volemia with central venous pressure (CVP) < 6 cm of fluid and maintain good renal flow. In critically ill patients, central venous access is recommended for preload evaluation and monitoring [18].
Early, vasoactive cardiotonic drugs should be introduced to maintain hemodynamic conditions and prevent shock, such as norepinephrine (from 0.01 to 1.0 μg/kg/min), which allows their use in a concentrated solution, allowing a low volume of infusion. As a second option, dopamine (2–5 μg/kg/min at dopa dose and 5–10 μg/kg/min at beta dose), both IV, should be used continuously. Dobutamine (8–15 μg/kg/min) should be reserved for refractory cases, in combination with more than one vasoactive drug when decreased myocardial performance is suspected, considering its use alone in the presence of severe hypotension may precipitate cardiac arrhythmias. When these drugs are not available, adrenaline and phenylephrine are used as second-choice drugs [18].
In more critically ill patients, continuous hemodynamic and ventilatory support and monitoring is required. Oxygen supply should be administered ensuring arterial saturation of at least 90% in patients who require it. In cases of mild respiratory failure and stable clinical status, early noninvasive ventilation (bilevel positive airway pressure ventilatory support [BIPAP)/continuous positive airway pressure (CPAP)] may be applied. Patients with more accentuated respiratory distress and O2 saturation < 80%, with signs of respiratory fatigue and chest X-ray compatible with severe ARDS, should be assisted with invasive (mechanical) ventilatory assistance. In this condition, PEEP (positive end-expiratory pressure) between 10 and 18 cm3 of H2O should be established in an attempt to reduce edema and the risk of pulmonary bleeding. In mechanical respiratory assistance, the controlled pressure mode is used, adjusting the inspiratory pressure so that the inspiratory peak of 35–40 cm3 is not exceeded, maintaining an adequate CO2 exchange (35–45 cm3) [18].
In the controlled volume modality, whenever possible, the tidal volume can be adjusted to 5–7 mL/kg of body weight, in an attempt to control with FiO2 < 60%, varying as necessary [18].
In cases of HFRS, in addition to the hemodynamic support, the maintenance of the hydroelectrolytic balance is crucial [28, 56]. The use of nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs, should be avoided [56].
In patients with acute renal failure with hemodynamic stability, intermittent hemodialysis is usually the first-choice procedure [28, 56]. In critically ill patients (multiorgan dysfunction syndrome, pulmonary edema, fluid overload, hydroelectrolytic disorders, encephalopathy, and acute renal failure), continuous renal replacement therapy is indicated [28, 56].
Antiviral drugs such as ribavirin and interferon-α have been used to treat both HCPS and HFRS, but scientific evidence suggests that the therapeutic effectiveness of these drugs is ideal when started prophylactically or at a very early stage of the disease [28, 56].
Recent evidence has indicated Icatibant (a drug used for the treatment of acute hereditary angioedema attacks), which acts as a bradykinin type 2 receptor antagonist, reducing the increase in vascular permeability and inhibiting vasodilation, as a possible therapeutic option in severe cases of hantavirus infection [56].
Finally, the isolation of the patient under barrier protection conditions (apron, gloves, and mask with N95 filters) is recommended, considering the reports of person-to-person transmission already reported in the literature [68].
14.11 Epidemiological Surveillance Actions
Cases of hantavirus infections should be compulsorily notified to the Brazilian Ministry of Health, and it is the obligation of health professionals to immediately report suspected cases. Every case of hantavirus infection should be investigated immediately after the notification, assessing the need for relevant control measures [17, 18].
The confirmatory diagnosis of a hantavirus infection case should consider the clinical, epidemiological, and laboratory diagnosis; thus, the Brazilian Ministry of Health adopts the following case definitions [17, 18]:
14.11.1 Suspected Case
-
Patient with fever >38 °C, myalgia and headache, and signs/symptoms of acute respiratory failure of undetermined etiology in the first week of the disease or
-
Patient with acute illness, with acute respiratory failure, progressing to death in the first week of the disease or
-
Patient with fever >38 °C, myalgia, and headache who has been exposed to a risk situation,∗ related or not to laboratory-confirmed cases
-
1.
Exposure to at-risk activities for hantavirus infection within 60 days prior to symptom onset: (a) deforestation, tree cutting, logging; (b) plowing, planting, or harvesting in the field; (c) grain transportation, storage, and milling; (d) storing or handling bales of hay, firewood, or similar; (e) cleaning barns or other similar constructions (greenhouses, granaries, storerooms, and silos); (f) cleaning of agricultural machinery; (g) entering, resting, and/or cleaning of residences or any other type of construction, occupied or not, regardless of the time; and (h) exposure to rural and/or wild environment in professional or leisure activities (hunting, fishing, ecotourism, military training, and scientific research).
-
2.
Existence of population of wild rodents and/or favorable environmental conditions for its establishment, in places frequented by the patient: (a) direct contact and/or presence of live/dead wild rodents or their excreta/traces (stool, urine, and/or urine smell); (b) presence of Brachiaria sp. grass; (c) abandoned fields, unoccupied grasslands; (d) change in the agricultural profile or other episodic natural phenomena that alter the availability of food (grains) for wild rodents, such as fruiting of native trees and flowering of bamboo plants [69]; (e) environmental factors that cause the displacement of rodents to the homes or surrounding human dwellings, such as deforestation, burning, floods, among others; and (f) climate changes and episodic natural phenomena with direct effects on the rodent population.
It is noteworthy that in Brazil, although HCPS is the predominant clinical pattern, there are studies that confirm the circulation of the Seoul virus in both humans and urban rodents of the genus Rattus, in addition to the description of cases with manifestations compatible with HFRS, clinically indistinguishable, and probably underdiagnosed in the presence of suspected cases of leptospirosis [7,8,9,10]. Therefore, infections caused by this virus should be suspected in patients with acute febrile syndrome, thrombocytopenia and proteinuria, or acute renal failure, with exposure to urban rodents [70].
14.11.2 Confirmed Case
14.11.2.1 According to Laboratory Criteria
Suspected case with the following laboratory test results:
-
Reactive serology for hantavirus-specific serum antibodies of the IgM class or
-
Positive tissue immunohistochemistry (identification of hantavirus-specific antigens)or
-
Positive RT-PCR for hantavirus
14.11.2.2 According to Epidemiological Clinical Criteria
-
Individual with a clinical condition of acute respiratory failure or acute renal failure who has died, without collection of specimens for specific tests, and who has been to areas of known hantavirus transmission or been exposed to the same at-risk situation as laboratory-confirmed patients in the last 60 days.
14.11.3 Discarded Case
-
Any suspected case that during the investigation has a laboratory-confirmed diagnosis of another disease or does not meet the previously defined confirmation criteria.
In parallel with the investigation of the human case, environmental surveillance activities are triggered, called eco-epidemiological surveillance actions, which imply activities at the probable site of infection, aimed at identifying the prevalent rodent species and, among them, determining the probable reservoir and the circulating hantavirus variant [18, 71]. These studies aim to expand the knowledge about the epidemiological behavior of hantavirus infections in a given area, contribute to the knowledge about the natural history of the disease, and assist decision-making regarding prevention and control actions [18].
Serological studies on wild rodents have been carried out since 1993, when the first cases of hantavirus were detected. Due to these studies, the identification of new hantavirus reservoirs, new genera and species of wild rodents, and new variants has been systematically performed [17, 18].
Serological surveys in human populations may also support the assessment and recognition of transmission areas, and studies carried out in Brazil have reported a prevalence of between 2% and 18%, depending on the study site and age group investigated. The most interesting data from surveys performed in Brazil are from Badra et al. [72], who retrospectively analyzed (1987–1990) samples from a blood bank of residents of the municipality of Cacia dos Coqueiros – state of São Paulo, and found a prevalence rate of around 5%, even before the confirmation of the first cases of the disease in the Americas.
14.12 Prevention and Control
The prevention of hantavirus infection is based on the use of measures that prevent human contact with wild and urban rodents and their excreta (waste eliminated from the organism) [73]. Protective equipment is recommended for individuals who work in environments with rodents (gloves, goggles, and masks with PFF3 filter) [18].
The control measures should include actions that prevent rodents from coming close, such as mowing the land around the house, adequately disposing of existing debris, keeping food stored in closed, rodent-proof containers, and other measures that prevent the interaction between man and wild and urban rodents, in places where the presence of these animals is known [18].
References
Mertz GJ, Hielle BL, Bryan RT. Hantavirus infection. Dis Mon. 1998;44:85–138.
Krüger D, Ulrich R, Lundkvist AA. Hantavirus infections and their prevention. Microbes Infect. 2001;3:1129–44.
Zeier M, Handermann M, Bahr U, et al. New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention. Virus Genes. 2005;30:157–80.
Lee HW, Lee P, Johnson KM. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever. From wild urban rats. J Infect Dis. 1982;146:638–44.
Clement JP. Hantavirus. Antivir Res. 2003;57:121–7.
Khan AS, Young JC. Hantavirus pulmonary syndrome: at the crossroads. Curr Opin Infect Dis. 2001;14:205–9.
Leduc JW, Smith GA, Pinheiro FP, et al. Isolation of a Hantaan-related virus from Brazilian rats and serologic evidence of its widespread distribution in South America. Am J Trop Med Hyg. 1985;34:810–5.
Hindrichsen S, Medeiros de Andrade A, Clement J, et al. Hantavirus infection in Brazilian patients from Recife with suspected leptospirosis. Lancet. 1993;341:50.
Iversson LB, Travassos da Rosa APA, Rosa MDB, et al. Infecção humana por hantavírus no sul e sudeste do Brasil. Revista de Assistência Médica Brasileira. 1994;40:85–92.
Costa F, Porter FH, Rodrigues G, et al. Infections by Leptospira interrogans, Seoul vírus, and Bartonella spp. among Norway rats (Rattus norvegicus) from the urban slum environment in Brazil. Vector Borne Zoonotic Dis. 2014;14:33–40.
Nichol ST, Spiropoulou CF, Morzunov S, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–7.
Childs JE, Ksiazek TG, Spiropoulou CF, et al. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J Infect Dis. 1994;169:1271–80.
Hantavirus en las Américas: guía para el diagnóstico, el tratamiento, la prevención y el control. Revista Española de Salud Pública. 1999;73:647.
Silva MVD, Vasconcelos MJ, Hidalgo NTR, et al. Hantavirus pulmonary syndrome: report of the first three cases in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 1997;39:231–4.
Childs JE, Mills JN, Glass GE. Rodent-borne hemorrhagic fever viruses: a special risk for mammalogists? J Mammal. 1995;6:664–80.
de Oliveira RC, Guterres A, Fernandes J, et al. Hantavirus reservoirs: current status with an emphasis on data from Brazil. Viruses. 2014;6:1929–73.
da Saúde M. Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços. Guia de Vigilância em Saúde: volume único. 2ª ed. Brasília: Ministério da Saúde; 2017; 705p
da Saúde M. Manual de vigilância, prevenção e controle das hantaviroses. Departamento de Vigilância Epidemiológica. Ministério da Saúde: Brasília; 2013. 94 p.
Campos GM, de Sousa RLM, Badra SJ, et al. Serological survey of hantavirus in Jardinopolis County, Brazil. J Med Virol. 2003;71:417–22.
de Oliveira SV, Fonseca LX, Barros PMR, et al. Análise do perfil epidemiológico da hantavirose no Brasil no período de 2007 a 2012. Revista de Patologia Tropical. 2014;43:131–42.
Firth C, Tokarz R, Simith DB, et al. Diversity and distribution of hantaviruses in South America. J Virol. 2012;86:13756–66.
Willemann MCA, Oliveira SV. Risk factors associated with hantavirosis fatality: a regional analysis from a case-control study in Brazil. Rev Soc Bras Med Trop. 2014;47:47–51.
Nunes ML, Oliveira SV, Elkhoury MDR, et al. Evidence of hantavirus circulation in a silent area of the Amazon region. Revista Pan-Amazônica de Saúde. 2015;6:63–7.
Mills JN, Childs JE. Rodent-borne hemorrhagic fever viruses. In: Infectious diseases of wild mammals: Wiley; 2008. p. 254–69.
Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J lnfect Dis. 1978;137:298–308.
Ferreira MS. Hantaviruses. Rev Soc Bras Med Trop. 2003;36:81–96.
Krautkramer E, Zeier M, Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int. 2013;83:23–7.
Jiang H, Du H, Wang LM, et al. Hemorrhagic fever with renal syndrome: pathogenesis and clinical picture. Front Cell Infect Microbiol. 2016;6:1.
Lin XD, Guo WP, Wang W, et al. Migration of Norway rats resulted in the worldwide distribution of Seoul hantavírus today. J Virol. 2012;86:972–81.
Padula PJ, Martinez VP, Cueto GR, et al. Caracterizacion genética parcial del hantavírus Seoul en ratas provenientes de Buenos Aires, Argentina, y la generacion de un antigeno a partir de la nucleoproteína recombinante del vírus Seoul. Revista Pan-Amazônica de Saúde. 2010;1:97–103.
Mcelhinney LM, Marston DA, Pounder KC, et al. High prevalence of Seoul hantavirus in a breeding colony of pet rats. Epidemiol Infect. 2017;145:3115–24.
Kerins JL, Koske SE, Kazmierczak J, et al. Outbreak of Seoul vírus among rats and rat owners – United State and Canada, 2017. Morb Mortal Wkly Rep (MMWR). 2018;67:131–4.
Hjelle B, Torres-Pérez F. Hantaviruses in the Americas and their role as emerging pathogens. Viruses. 2010;2:2559–86.
Nelson R, Cañate R, Pascale JM, et al. Confirmation of Choclo virus as the cause of hantavirus cardiopulmonary syndrome and high serum antibody prevalence in Panama. J Med Virol. 2010;82:1586–93.
Milazzo ML, Duno G, Utrera A, et al. Natural host relationships of hantaviruses native to western Venezuela. Vector Borne Zoonotic Dis. 2010;10:605–11.
Saasa N, Sánchez-Hernández C, de Lourdes Romero-Almaraz M, et al. Ecology of hantaviruses in Mexico: genetic identification of rodent host species and spillover infection. Virus Res. 2012;168:88–96.
Butler JC, Peters CJ. Hantaviruses and hantavirus pulmonary syndrome. Clin Infect Dis. 1994;19:387–95.
Toro J, Vega JD, Khan AS, et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg Infect Dis. 1998;4:687.
Londoño AF, Levis S, Rodas JD. Hantavirus como agentes emergentes de importancia en Suramérica. Biomedica. 2011;31(3):451–64.
Oliveira SV, Fonseca LX, Araújo-Vilges KM, et al. Vulnerability of Brazilian municipalities to hantavirus infections based on multi-criteria decision analysis. Emerg Themes Epidemiol. 2015;12:15.
Mattar S, Garzon D, Tadeu L, et al. Serological diagnosis of hantavírus pulmonary syndrome in a febrile patient in Colombia. Int J Infect Dis. 2014;25:201–3.
Padula P, Martinez VP, Bellomo C, et al. Pathogenic hantaviruses, northeastern Argentina and eastern Paraguay. Emerg Infect Dis. 2007;13:1211.
Wells RM, Sosa Estani S, Yadon ZE, et al. An unusual hantavírus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg Infect Dis. 1997;3:171–4.
Peters CJ. Hantavirus pulmonary syndrome in the Americas. In: Emerging infections 2: American Society of Microbiology; 1998. p. 17–64.
Peters CJ, Simpson GL, Levy H. Spectrum of hantavírus infection: hemorrhagic fever with renal syndrome and hantavírus pulmonary syndrome. Annu Rev Med. 1999;50:531–45.
Castillo C, Naranjo J, Sepúlveda A, et al. Hantavirus pulmonary syndrome due to Andes vírus in Temuco, Chile: clinical experience with 16 adults. Chest. 2001;120:548–54.
Ferrer JF, Jonsson CB, Esteban E, et al. High prevalence of hantavirus infection in Indian communities of the Paraguayan and Argentinean Gran Chaco. Am J Trop Med Hyg. 1998;59:438–44.
Padula P, Colavecchia S, Martínez P, et al. Genetic diversity, distribution and serological features of hantavirus infection in five countries in South America. J Clin Microbiol. 2000;38:3029–35.
Weissenbacher MC, Cura E, Segura EL, et al. Serological evidence of human hantavirus infection in Argentina, Bolivia and Uruguay. Medicina (B Aires). 1996;56:17–22.
Oliveira SV, Escobar LE, Peterson AT, et al. Potential geographic distribution of hantavirus reservoirs in Brazil. PLoS One. 2013;8:e85137.
de Oliveira RC, Cordeiro-Santos M, Guterres A, et al. Rio Mamoré virus and hantavirus pulmonary syndrome, Brazil. Emerg Infect Dis. 2014;20:1568.
Lee JS. Clinical features of hemorrhagic fever with renal syndrome in Korea. Kidney Int. 1991;40:88–93.
Settergren B, Juto P, Trollfors B, et al. Clinical characteristics of nephropathia epidemica in Sweden: prospective study of 74 cases. Rev Infect Dis. 1991;11:949–55.
Mattar S, Guzmán C, Figueiredo LT. Diagnosis of hantavirus infection in humans. Expert Rev Anti-Infect Ther. 2015;13:939–46.
Muranyi W, Bahr U, Zeier M, et al. Hantavirus infection. J Am Soc Nephrol. 2005;16:3669–79.
Mustonen J, Outinen T, Laine O, et al. Kidney disease in Puumala hantavírus infection. Infect Dis. 2017;49:321–32.
Clement J, Kuypers D, Meijers B, Van Ranst M. Hantavirus infection with renal involvement do not result in chronic renal diseases or end-stage renal failure. J Nephrol Renal Dis. 2017;1:1.
Mustonen J, Makela S, Outinen T, et al. The pathogenesis of nephropathia epidêmica: new knowledge and unanswered questions. Antivir Res. 2013;100:589–604.
Riquelme R, Riquelme M, Torres A, et al. Hantavirus pulmonary syndrome, southern Chile. Emerg Infect Dis. 2003;9:1438–43.
Verity R, Prasad E, Grimsrud K, et al. Hantavirus pulmonary syndrome in northern Alberta, Canada: clinical and laboratory findings for 19 cases. Clin Infect Dis. 2000;31:942–6.
Ketai LH, Kelsey CA, Jordan K, et al. Distinguishing hantavirus pulmonary syndrome from acute respiratory distress syndrome by chest radiography: are there different radiographic manifestations of increased alveolar permeability? J Thorac Imaging. 1998;13:172–7.
Schmaljohn CS, Nichol ST. Hantaviruses. Curr Top Microbiol Immunol. 2001;256:1–196.
Mustonen J, Makela S, Outinen T, et al. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antivir Res. 2013;100:589–604.
Krautkramer E, Zeier M. Old World hantavírus: aspects of pathogenesis and clinical course of acute renal failure. Virus Res. 2014;187:59–64.
Zaki SR. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552.
Nolte KB, Feddersen RM, Foucar K, et al. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26:110–20.
Gnemmi V, Verine J, Vrigneaud L, et al. Microvascular inflammation and acute tubular necrosis are major histologic features of hantavírus nephropathy. Hum Pathol. 2015;46:827–35.
Martinez VP, Bellomo C, San Juan J, et al. Person-to-person transmission of Andes virus. Emerg Infect Dis. 2005;11:1848.
Oliveira SV, Folster I, Zeccer S, et al. Investigação de ratada associada a florescimento e frutificação de taquaras em São Francisco do Sul, Santa Catarina, Brasil, 2012. Revista Baiana de Saúde Pública. 2014;37:1071.
Clement J, Neild G, Hinrichsen SL, et al. Urban leptospirosis versus urban hantavírus infection in Brazil. Lancet. 1999;354:2003–4.
Pereira LE, Souza LTM, Souza RP, et al. Histórico da Vigilância Ecoepidemiológica do Hantavírus no Brasil. Revista CIP. 1999;3:5–12.
Badra SJ, Maia FGM, Figueiredo GG, et al. A retrospective serologic survey of hantavirus infections in the county of Cássia dos Coqueiros, state of São Paulo, Brazil. Rev Soc Bras Med Trop. 2012;45:468–70.
Oliveira SV, Lassance CL, Nascimento GL, et al. Conhecimentos, atitudes e práticas sobre hantavirose em um assentamento rural de Planaltina-Distrito Federal, 2011. Scientia Plena. 2012;8:6.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Oliveira, S.V., Faccini-Martínez, Á.A. (2020). Hantavirus Infection and the Renal Syndrome. In: Bezerra da Silva Junior, G., De Francesco Daher, E., Barros, E. (eds) Tropical Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-44500-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-44500-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44499-0
Online ISBN: 978-3-030-44500-3
eBook Packages: MedicineMedicine (R0)