Abstract
Nasal obstruction in the pediatric patient can lead to decreased quality of life and contribute to sleep disordered breathing. In severe cases with prolonged obligate mouth breathing, it may even lead to dental and craniofacial abnormalities. In children who present with nasal obstruction, a thorough physical exam and nasal endoscopy should be performed with particular attention to the nasal septum and inferior turbinates. If symptoms persist despite appropriate medical therapy, surgery should be considered. A septoplasty may be performed safely and effectively with good evidence that the procedure does not affect craniofacial development if performed with meticulous surgical techniques. Inferior turbinate reduction may be performed in a variety of ways, although most modern techniques avoid destruction of the overlying mucosa.
Similar content being viewed by others
Keywords
Introduction
As in the adult patient, nasal obstruction may significantly affect quality of life and contribute to other disease processes such as obstructive sleep apnea (OSA) in the pediatric patient. Although there are multiple possible causes of nasal obstruction , structural issues such as a nasal septal deviation (NSD) and inferior turbinate hypertrophy (ITH) are frequently discovered in the workup of a child who presents with nasal obstruction. Nasal obstruction from any cause often leads to obligate mouth breathing which has been associated with dental malocclusion and abnormalities of craniofacial development [1,2,3]. Obligate mouth breathing caused specifically by NSD has also been shown to be associated with craniofacial and dental anomalies [4, 5]. The historical controversies over concern for the effects of pediatric septoplasty on craniofacial growth may cause apprehension for the surgeon. However, recent research demonstrates that septoplasty performed using meticulous technique does not result in long-term craniofacial growth abnormalities. Although septoplasty and inferior turbinate reduction have no role in the management of chronic sinusitis [6], they have been shown to be very effective in treating nasal obstruction in the pediatric patient.
Anatomy
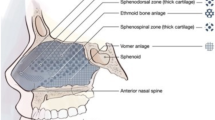
The septum is made of mucoperichondrial and mucoperiosteal flaps that envelope the quadrangular cartilage and bony septum, comprised of the perpendicular plate of the ethmoid bone, the vomer, and the crests of the maxillary and palatine bones. The main difference between the pediatric and adult septum is in the proportion of the septum made up of cartilage. An anatomic study has shown that the cartilaginous portion of the septum reaches adult dimensions by 2 years of age, with subsequent growth due to expansion of the bony parts of the septum [7]. The inferior turbinate consists of a bony core covered by mucosa and vascularized erectile submucosal tissue. It is formed by the embryonic maxilloturbinal, which develops as a projection from the lateral nasal wall. Unlike the other turbinates, the inferior turbinate is not considered to be of ethmoid origin embryologically. The inferior turbinate attaches to the lateral nasal wall just below the middle turbinate, from just posterior to the pyriform aperture anteriorly to just anterior to the choanae posteriorly [8].
Evaluation of the Septum and Inferior Turbinate in the Pediatric Patient
A thorough head and neck exam with particular attention to anterior rhinoscopy and a thorough nasal endoscopy is optimal to determine the cause or causes of nasal obstruction. Although NSD and ITH commonly contribute to pediatric nasal obstruction, adenoid hypertrophy is often found concurrently. Furthermore, congenital nasal masses must be ruled out. Not all practitioners perform routine nasal endoscopy; in a survey of pediatric otolaryngologists on the evaluation and management of the pediatric patient with bilateral nasal obstruction, plain film was used more frequently in younger age groups while fiberoptic nasal endoscopy was used more often in older age groups [9]. The authors hypothesized that this was because fiberoptic endoscopy is more difficult to perform and adenoid hypertrophy is more likely in the younger patient (ages 3–6). If adenoid hypertrophy is suspected, a lateral neck plain film may be helpful. If the examination raises concern for a nasal mass, computed tomography (CT) scan and magnetic resonance imaging (MRI) should also be performed.
Full evaluation of the pediatric patient who presents with nasal obstruction must also consider allergic and nonallergic rhinitis. Allergic rhinitis is among the most common chronic conditions of childhood [10] with well-defined clinical symptoms and management guidelines [11, 12]. In a study of children and adolescents with moderate-to-severe persistent allergic rhinitis who were treated with daily intranasal steroids and oral antihistamines or leukotriene receptor antagonists for at least 2 months, nonresponders to medical therapy showed a higher prevalence of objective NSD and severe ITH compared to patients who responded to medical therapy. This study highlights the importance of appropriate follow-up, a thorough physical exam, and a broad differential diagnosis in pediatric patients who present with nasal obstruction [13].
Consideration must also be given to the possibility of concurrent sleep disordered breathing. It has been shown that nasal obstruction may contribute to OSA [14]. Although adenotonsillar hypertrophy is the most common cause of OSA in children, a significant portion of children have persistent apnea or hypopnea after tonsillectomy and adenoidectomy [15]. A subset of these patients has nasal obstruction due to an NSD and/or ITH, indicating the importance of a thorough exam prior to embarking on surgery.
Pediatric Septoplasty: Effect on Facial Growth
Although septoplasty is one of the most common procedures performed by otolaryngologists on adults, it has been a controversial area in the pediatric population because early animal studies demonstrated craniofacial growth abnormalities following septal resection. In a study from 1858, examining anatomic parameters after resection of the cartilaginous nasal septum in growing animals, the hard palate was found to be significantly shorter in the anterior-to-posterior direction [16]. In a study several decades later, Landsberger resected the anterior septum in a young canine model and discovered that the nasal cavity floor was higher than normal 6 months later [17], resulting in the hypothesis that growth of the septum affected the position of the hard palate. A later study on the effects of resection of the cartilaginous septum and mucoperichondrium in growing rabbits found underdevelopment of the nasal and premaxillary bones with the extent and severity of deformity proportional to the extent of the septal defect [18]. More recently, animal studies have found no effect on facial growth if the mucoperichondrial flaps were preserved during septoplasty, with studies performed on both canine pups [19] and growing ferrets [20].
Following these promising findings with mucoperichondrial flap preservation in animal models, human studies began appearing which confirmed the lack of effect on craniofacial growth. Both Jugo [21] and Triglia et al. [22] performed external septoplasty in children and did not find any serious alterations on craniofacial growth based on subjective visual assessment. Studies utilizing anthropometric measurements provided a more objective analysis. Bejar et al. [23] compared postoperative anthropometric measurements in 28 children who underwent external septoplasty to normative data and found that most measurements were similar to normal averages. Although the nasal dorsal length was decreased in this cohort postoperatively, it is unclear if this was attributable to surgery as measurements were not routinely made preoperatively. To build on these findings, El-Hakim et al. [24] compared preoperative to postoperative measurements in 26 pediatric patients undergoing external septoplasty. Although nasal dorsum length and nasal tip protrusion were decreased postoperatively, the differences were not statistically significant. With regard to conservative endonasal septoplasty, one study of 44 pediatric patients found no significant differences in anthropometric measurements compared to normal values when taken at an average of 12.2 years following surgery [25].
Pediatric Septoplasty: Indications
Based on the available research, current evidence suggests that pediatric septoplasty with careful preservation of the mucoperichondrial flaps can be performed without altering craniofacial growth. In addition, there is evidence that untreated obligate mouth breathing may lead to dental malocclusion and craniofacial growth disturbance. Therefore, a septoplasty should be considered in the child with an NSD that is associated with obligate mouth breathing or obstructive sleep apnea. To date, there is no consensus based on the published data that defines the minimum age to perform a septoplasty. However, several authors have advocated a minimum age of five [26] or six [23, 27] years in children with severe nasal obstruction caused by NSDs. Other studies have advocated for closed reduction of severe NSDs in the neonatal period as malocclusion was frequently found if left untreated [5, 28]. In neonates, NSDs can occur due to trauma in utero or during birth and may be associated with important clinical implications including failure to thrive or respiratory distress. These can be ameliorated with closed reduction within the first few weeks of life. Finally, with the expansion of endoscopic endonasal skull base surgery in the pediatric population, septoplasty may be indicated for access to certain skull base pathologies [29].
Pediatric Septoplasty: Outcomes
Several studies have evaluated outcomes after pediatric septoplasty. Dispenza et al. performed a retrospective study of 46 patients (aged 4–12) who underwent endonasal septoplasty or closed septorhinoplasty and were followed for an average of 10 years [30]. Of the 16 patients with isolated NSDs, only 1 patient (6.3%) developed a recurrence postoperatively. Of the remaining patients with combined nasal and septal deformity, a lower rate of recurrent NSD was identified in those treated with septorhinoplasty (14.7%) relative to septoplasty alone (25%), although no statistical analysis was performed. In a study evaluating postoperative quality of life, Yilmaz et al. followed 35 patients with a mean age of 13.4 years for 12 months after endonasal septoplasty and found significant improvements in both nose obstruction symptom evaluation (NOSE) and visual analog scale (VAS) [31]. A second study of 28 patients found significant improvements postoperatively in the VAS as well as in the Sinus and Nasal Quality of Life Survey (SN-5), a quality of life instrument validated in the pediatric population [32].
Pediatric Septoplasty: Technique
A variety of operative techniques can be utilized to correct an NSD in the pediatric patient. Although closed septal repositioning does not have much use in managing a deviated nasal septum in the adult patient, it may be efficacious in the pediatric patient, particularly in the setting of an acutely displaced septum as a result of trauma [26]. A Boies elevator or Asch forceps may be used in this situation. Targeted endoscopic septoplasty may also be performed. In the setting of small, isolated spurs, the spur may be resected without flap elevation [26], although resection of mucoperichondrium must be kept to a minimum. The majority of septal spurs can be managed safely via an endoscopic approach with a small mucoperichondrial flap raised over the deviated bone and cartilage only (Figs. 19.1 and 19.2). Alternatively, a standard Killian or hemitransfixion incision may be made and a mucoperichondrial flap raised. Once the deviated cartilage is isolated, straightening may be achieved with relaxing incisions along the convex side of the deviation. If cartilage needs to be removed to achieve an adequate nasal airway, it is important to reimplant any straight pieces of bone and cartilage [24] or to flatten or crush pieces of deviated cartilage and reimplant them between the mucoperichondrial flaps. At the conclusion of the procedure, an absorbable quilting suture and/or Silastic splints may be used to reapproximate the mucoperichondrial flaps.
An endoscopic view of the left nasal cavity demonstrating a septal deviation compromising the nasal airway. This deviation can be removed safely via an endoscopic approach with a small mucoperichondrial flap raised over the deviated bone and cartilage only. Only the deviated cartilage and bone (red-checked area) should be removed. Any straight pieces of bone and cartilage are replaced between the mucoperichondrial flaps. The septal bone and cartilage above the white line are not removed. (S Septum, IT Inferior turbinate)
Surgery on the Inferior Turbinate
ITH is a very common cause of pediatric nasal obstruction (Fig. 19.3). Often found concurrently with allergic rhinitis, medical therapy may include intranasal corticosteroid sprays, oral leukotriene receptor antagonists, antihistamines (oral or intranasal), and immunotherapy in the appropriate patient. If medical treatments fail to achieve symptomatic relief, surgery on the inferior turbinates may be performed, with the goal of expanding the nasal airway while preserving the mucosa of the turbinate to minimize crusting and preserve function. Inferior turbinate reduction may be performed as an isolated procedure or in conjunction with adenotonsillectomy, adenoidectomy, endoscopic sinus surgery, or septoplasty.
There are several surgical options to treat inferior turbinate hypertrophy . Partial and total turbinectomy were once the procedures of choice [33] but have largely been replaced by mucosal-sparing techniques due to the concerns for postoperative pain, crusting, bleeding, and atrophic rhinitis [34]. Lasers have also been used for destruction of hypertrophic mucosa of the inferior turbinate , most commonly with the carbon dioxide (CO2) and neodymium:yttrium-aluminum garnet (Nd:YAG) lasers. However, this technique has largely fallen out of favor given the risks of persistent crusting, atrophy, and synechiae formation [35].
Results from early studies have led to the adoption of mucosal-sparing techniques. Submucosal resection is a mucosal-sparing option in which the submucosal tissue is removed with or without bone removal. Monopolar electrocautery with Bovie may be performed in the submucosal tissue but creates high temperatures and often thermal damage to the mucosal layer. A more recent development is radiofrequency ablation, which uses a submucosal radiofrequency delivered by bipolar current to create a plasma field that ablates soft tissue and creates necrosis at a lower temperature, therefore preserving the overlying mucosa. As the area of necrosis heals, the lesion contracts, leading to a reduction in size of the inferior turbinate [36]. In addition to research demonstrating long-term benefits in adults [37], it has been shown to be effective and safe in the pediatric population [38]. Microdebrider-assisted inferior turbinoplasty (MAIT) is another option [39]. After the inferior turbinate is infiltrated with local anesthetic, a vertical incision is made at the head of the inferior turbinate. A submucosal tunnel is then created with sharp dissection, and a straight microdebrider is applied through the incision. Lower-profile microdebrider blades specifically designed for this procedure have been developed (Inferior Turbinate Blade; Medtronic Corporation, Minneapolis, MN; Fig. 19.4). The incision site is then cauterized if needed, and typically no nasal packing is necessary.
Multiple comparisons of the various surgical techniques have been performed for managing inferior turbinate hypertrophy in the adult patient. A systematic review and meta-analysis of studies comparing radiofrequency ablation and MAIT found patient improvement with both techniques, although the largest, highest-quality studies favored MAIT [40]. For the pediatric population, there have been relatively few studies evaluating outcomes of inferior turbinate surgery. A review of the literature performed in 2009 identified 11 articles in which turbinate surgery was performed in pediatric patients with nasal congestion refractory to medical management [41]. Each article identified in this review evaluated techniques popular at the time of the article’s publication, with the earlier studies reporting on partial or total turbinectomy and the more recent studies reporting on radiofrequency ablation and microdebrider use. Overall, 50–94% of participants improved subjectively, although the heterogeneity of the outcome measures did not allow for meta-analysis. Since that time, two other studies have been performed. Cheng et al. evaluated 51 children with obstructive sleep apnea and nasal congestion due to persistent severe allergic rhinitis refractory to medical therapy, of which 28 underwent adenotonsillectomy (AT) alone and 23 underwent adenotonsillectomy with concurrent MAIT [42]. When compared to the cohort that underwent AT alone, the cohort that underwent AT and MAIT showed significantly greater improvements postoperatively with respects to apnea-hypopnea index, acoustic rhinometry, and subjective quality of life. The second study was a retrospective review from a single academic institution of 107 children who underwent surgery on the inferior turbinate [43]. Procedures included radiofrequency ablation (67.3%), MAIT (17.8%), and partial turbinate resection (19.6%). Revision inferior turbinate surgery was performed in 7.5% of all patients with no significant difference between the surgical techniques used at the initial procedure. Based on a telephone survey utilizing a 5-point Likert scale taken at a median of 4.55 years after the procedure, the authors found that 70% of patients were satisfied or extremely satisfied with the procedure, with no difference between the various surgical techniques.
Although there is limited evidence on the long-term benefits of inferior turbinate surgery in pediatric patients, a survey of pediatric otolaryngologists discovered that 81% of respondents performed the procedure, with 47% of preferring coblation, 16% using MAIT primarily, and the remainder preferring other techniques [44]. This high rate is likely due at least in part to the safety of pediatric turbinate surgery, with an overall complication rate of around 4% based on the largest review [41]. The complications in this study consisted of intranasal synechiae (62%) and postoperative epistaxis (34%). Inferior turbinate reduction has also not been shown to increase the complication rate when performed concurrently with adenoidectomy [45] or AT [46].
Conclusion
Septoplasty and inferior turbinate reduction may be performed safely and effectively in the pediatric patient with nasal obstruction due to a deviated nasal septum and inferior turbinate hypertrophy refractory to medical therapy. Historical concerns regarding craniofacial developmental abnormalities have been alleviated, as recent publications have demonstrated no effect on facial growth if mucoperichondrial septal flaps are preserved. Proper surgical technique and patient selection optimizes chances for a successful outcome and improved quality of life.
References
Linder-Aronson S. Adenoids: their effect on mode of breathing and nasal airflow and their relationship to characteristics of the facial skeleton and the dentition. A biometric, rhino-manometric and cephalometro-radiographic study on children with and without adenoids. Acta Otolaryngol Suppl. 1970;265:1–132.
Luzzi V, Di Carlo G, Saccucci M, et al. Craniofacial morphology and airflow in children with primary snoring. Eur Rev Med Pharmacol Sci. 2016;20(19):3965–71.
Bresolin D, Shapiro PA, Shapiro GG, Chapko MK, Dassel S. Mouth breathing in allergic children: its relationship to dentofacial development. Am J Orthod. 1983;83(4):334–40.
D’ascanio L, Lancione C, Pompa G, Rebuffini E, Mansi N, Manzini M. Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol. 2010;74(10):1180–3.
Pentz S, Pirsig W, Lenders H. Long-term results of neonates with nasal deviation: a prospective study over 12 years. Int J Pediatr Otorhinolaryngol. 1994;28(2–3):183–91.
Brietzke S, Shin J, Choi S, et al. Clinical consensus statement: pediatric chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;151(4):542–53.
Van Loosen J, Van Zanten GA, Howard CV, Verwoerd-Verhoef HL, Van Velzen D, Verwoerd CD. Growth characteristics of the human nasal septum. Rhinology. 1996;34(2):78–82.
Mynatt RG, Sindwani R. Surgical anatomy of the paranasal sinuses. In: Stucker FJ, de Souza C, Kenyon GS, Lian TS, Draf W, Schick B, editors. Rhinology and facial plastic surgery. Berline: Springer; 2009.
Kohlberg GD, Stewart MG, Ward RF, April MM. Evaluation and management of pediatric nasal obstruction: a survey of practice patterns. Am J Rhinol Allergy. 2016;30:274–8.
Izquierdo-Dominguez A, Valero AL, Mullol J. Comparative analysis of allergic rhinitis in children and adults. Curr Allergy Asthma Rep. 2013;13:142–51.
Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update. Allergy. 2008;63:8–160.
Montoro J, Del Cuvillo A, Mullol J, et al. Validation of the modified allergic rhinitis and its impact on asthma (ARIA) severity classification in allergic rhinitis children: the PEDRIAL study. Allergy. 2012;67:1436–42.
Marino-Sanchez FS, Valls-Mateus M, Ruiz-Echevarria K, Alobid I, Cardenas-Escalante P, Jimenez-Feijoo R, Lozano-Blasco J, et al. Nasal obstructive disorders induce medical treatment failure in pediatric persistent allergic rhinitis (the NODPAR study). Pediatr Allergy Immune. 2017;28:176–84.
Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest. 1986;90:324–9.
Lipton AJ, Gozal D. Treatment of obstructive sleep apnea in children: do we really know how? Sleep Med Rev. 2003;7:61–80.
Fick L. Uber die Ursachen der Knochenforman: Experimental Untersuchung. Wigand GH: Gottingen; 1858.
Landsberger R. Die triebende Krafte zur Dehnung und Streckung des Gesichtschadels. Zahnarztliche Runschau. 1929;23:978–90.
Sarnat BG, Wexler MR. Growth of the face and jaws after resection of the septal cartilage in the rabbit. Am J Anat. 1966;118:755–67.
Bernstein L. Early submucous resection of nasal septal cartilage: a pilot study in canine pubs. Arch Otolaryngol. 1973;97(3):273–8.
Cupero TM, Middleton CE, Silva AB. Effects of functional septoplasty on the facial growth of ferrets. Arch Otolaryngol Head Neck Surg. 2001;127(11):1367–9.
Jugo SB. Total septal reconstruction through decortication (external) approach in children. Arch Otolaryngol Head Neck Surg. 1987;113(2):173–8.
Triglia JM, Cannoni M, Pech A. Septorhinoplasty in children: benefits of the external approach. J Otolaryngol. 1990;19(4):274–8.
Bejar I, Farkas LG, Messner AH, Crysdale WS. Nasal growth after external septoplasty in children. Arch Otolaryngol Head Neck Surg. 1996;122:816–21.
El-Hakim H, Crysdale WS, Abdollel M, Farkas LG. A study of anthropometric measures before and after external septoplasty in children: a preliminary study. Arch Otolaryngol Head Neck Surg. 2001;127:1362–6.
Tasca I, Compadretti GC. Nasal growth after pediatric septoplasty at long-term follow-up. Am J Rhinol Allergy. 2011;25:e7–e12.
Christophel JJ, Gross CW. Pediatric Septoplasty. Otolaryngol Clin N Am. 2009;42:287–94.
Crysdale WS, Walker PJ. External septorhinoplasty in children: patient selection and surgical technique. J Otolaryngol. 1994;23:28–31.
Sooknundun M, Kacker SK, Bhatia R, Deka RC. Nasal septal deviation: effective intervention and long term follow-up. Int J Pediatr Otorhinolaryngol. 1986;12(1):65–72.
Chivukula S, Koutourousiou M, Snyderman CH, Fernandez-Miranda JC, Gardner PA, Tyler-Kabara EC. Endoscopic endonasal skull base surgery in the pediatric population. J Neurosurg Pediatr. 2013;11:227–41.
Dispenza F, Saraniti C, Sciandra D, Kulamarva G, Dispenza C. Management of nasoseptal deformity in childhood: long-term results. Auris Nasus Larynx. 2009;36:665–70.
Yilmaz MS, Guven M, Akidil O, Kayabasoglu G, Demir D, Mermer H. Does septoplasty improve the quality of life in children? Int J Pediatr Otorhinolaryngol. 2014;78:1274–6.
Lee VS, Gold RM, Parikh SR. Short-term quality of life outcomes following pediatric septoplasty. Acta Otolaryngol. 2017;137(3):293–6.
Segal S, Eviatar E, Berenholz L, Kessler A, Shlamkovitch N. Inferior turbinectomy in children. Am J Rhinol. 2003;17(2):69–73.
Nurse LA, Duncavage JA. Surgery of the inferior and middle turbinates. Otolaryngol Clin N Am. 2009;42(2):295–309.
Janda P, Stroka R, Baumgartner R. Laser treatment of hyperplastic inferior nasal turbinates: a review. Lasers Surg Med. 2001;28:404–14.
Utley DS, Goode RL, Hakim I. Radiofrequency energy tissue ablation for the treatment of nasal obstruction secondary to turbinate hypertrophy. Laryngoscope. 1999;109:683–6.
Bhattacharyya N, Kepnes LJ. Clinical effectiveness of coblation inferior turbinate reduction. Otolaryngol Head Neck Surg. 2003;129:365–71.
Bitar MA, Kanaan AA, Sinno S. Efficacy and safety of inferior turbinate coblation in children. J Laryngol Otol. 2014;128(Suppl 2):S48–54.
Friedman M, Tanyeri H, Lim J, Landsberg R, Caldarelli D. A safe, alternative technique for inferior turbinate reduction. Laryngoscope. 1999;109(11):1834–7.
Acevedo JL, Camacho M, Brietzke SE. Radiofrequency ablation turbinoplasty versus microdebrider-assisted turbinoplasty: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;153(6):951–6.
Leong SC, Kubba H, White PS. A review of outcomes following inferior turbinate reduction surgery in children for chronic nasal obstruction. Int J Pediatr Otorhinolaryngol. 2010;74:1–6.
Cheng PW, Fang KM, Su HW, Huang TW. Improved objective outcomes and quality of life after adenotonsillectomy with inferior turbinate reduction in pediatric obstructive sleep apnea with inferior turbinate hypertrophy. Laryngoscope. 2012;122(12):2850–4.
Arganbright JM, Jensen EL, Mattingly J, Gao D, Chan KH. Utility of inferior turbinoplasty for the treatment of nasal obstruction in children: a 10-year review. JAMA Otolaryngol Head Neck Surg. 2015;141(10):901–4.
Jiang ZY, Pereira KD, Friedman NR, Mitchell RB. Inferior turbinate surgery in children: a survey of practice patterns. Laryngoscope. 2012;122:1620–3.
Langille M, El-Hakim H. Pediatric inferior turbinoplasty with or without adenoidectomy: preliminary report on improvement of quality of life, symptom control, and safety. Otolaryngol Head Neck Surg. 2011;40:420–6.
Yuen SN, Leung PP, Funamura J, Kawai K, Roberson DW, Adil EA. Complications of turbinate reduction surgery in combination with tonsillectomy in pediatric patients. Laryngoscope. 2017;127:1920–3.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Soneru, C.P., Riley, C.A., Gudis, D.A. (2020). Septoplasty and Turbinate Reduction in Children. In: Ramadan, H., Baroody, F. (eds) Pediatric Rhinosinusitis. Springer, Cham. https://doi.org/10.1007/978-3-030-22891-0_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-22891-0_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22890-3
Online ISBN: 978-3-030-22891-0
eBook Packages: MedicineMedicine (R0)