Abstract
Open surgical repair using free grafts or pedicled skin flaps, called substitution urethroplasty, is the gold standard procedure for bulbar urethral strictures not suitable for excision and primary anastomosis. Buccal mucosa harvested from the inner cheek is an ideal urethral substitute and is the tissue most often used for substitution urethroplasty because of its compatibility with urethral tissue, the ease of harvesting and handling it. Since the start of widespread use of the buccal mucosa graft in substitution urethroplasty, the use of pedicled skin flaps have become less common. Most bulbar urethral strictures can be repaired by one-stage urethroplasty, and buccal mucosa graft onlay augmentation is the most preferred procedure. Onlay augmentation on the ventral side (ventral onlay) or dorsal side (dorsal onlay) has been widely used, and other options are dorsal inlay with ventral sagittal urethrotomy or lateral onlay with one-sided urethral dissection. The success rate is the same for the different graft positions. In bulbar urethral strictures with obliterative or nearly obliterative segments, either a two-sided dorsal plus ventral onlay augmentation or a combination of excision and primary anastomosis and onlay augmentation (augmented anastomotic urethroplasty) is the procedure of choice. In this chapter, a practical strategy of substitution bulbar urethroplasty mainly using buccal mucosa graft is described.
Similar content being viewed by others
Keywords
1 Background
Male anterior urethral stricture is one of the oldest urologic disorders and has an estimated prevalence of 0.6% in susceptible populations [1, 2]. Anterior urethral stricture, a fibrosis of the epithelial tissue and corpus spongiosum resulting in stenosis of the urethral lumen, is a relatively common urologic problem with various etiologies—typically including external trauma, iatrogenic factors, and genital lichen sclerosus (LS)—that decreases the urinary stream and adversely impacts not only patient-reported quality of life but also overall health status [3]. The bulbar urethra is the most common site of anterior urethral stricture [4, 5] and its etiology is idiopathic in about 40% of cases, iatrogenic in 35%, traumatic in 15%, and inflammatory in 10% [3].
The surgical management of bulbar urethral stricture has changed dramatically in the last several decades [6]. The long-term outcome of transurethral managements such as dilation and urethrotomy that have been widely used has been found to be poor, and urethroplasty has become standard treatment for urethral stricture [7]. The types of urethroplasty for bulbar urethral strictures are mainly categorized into two groups (Fig. 16.1). One is excision and primary anastomosis (EPA), which consists of resecting all fibrotic urethral segments and tension-free re-approximation of the proximal and distal urethral ends [8]. This group also includes non-transecting repair , which was described as an alternative to an EPA for very short bulbar strictures with minimal spongiofibrosis in order to preserve bulbar arteries and avoid sexual dysfunction resulting from corpus spongiosum transection [9,10,11]. The other group is substitution urethroplasty in which the fibrotic urethral segment is augmented or replaced by using grafts or flaps [12] and has become the gold standard procedure for urethral strictures that are not suitable for EPA. Although EPA is the preferred method of urethral reconstruction whenever possible because of its high success rate and durability compared to substitution urethroplasty [13,14,15], the length and location of the stricture and the size and elasticity of the urethral segment limit the use of EPA [16,17,18]. The amount of length that can be gained will depend on the anatomy of the individual male patient: the size of his penis and urethra, or particularly, the length and elasticity of the distal urethral segment [12]. Stricture in the proximal bulbar urethra can be bridged because the bulbar urethra can be fully elongated by full mobilization of it and separating the corpora can straighten the natural curve of the bulbar urethra and shorten its course [18]. For a longer stricture, however, excising the entire stricture and making an anastomosis without tension is impossible. When the stricture is in the more distal bulbar urethra, the gap that can be bridged is shorter and anastomotic urethroplasty is applicable only for short (probably within 1 cm) strictures because mobilizing the bulbar urethra beyond the region distal to the penoscrotal junction has a risk of causing chordee [18]. Moreover, some experts favor substitution urethroplasty even for bulbar strictures that can be managed by EPA because EPA can cause sexual dysfunction such as ejaculatory dysfunction, decreased glans sensitivity, and penile chordee and can decrease penile length [19,20,21]. Substitution urethroplasty is therefore the procedure of choice for a long stricture in the proximal bulbar urethra or a stricture of any length located anywhere from the distal bulbar urethra and for strictures in patients who worry about sexual dysfunction.
2 Advantage of Buccal Mucosa as a Urethral Substitute
A number of different tissue types—including penile skin, scrotal skin, extragenital skin, bladder mucosa, intestinal mucosa, and oral mucosa—have been used as urethral substitutes [22,23,24,25,26,27]. Traditionally, penile skin as a pedicled flap or as a free graft had been considered the most reliable material for reconstruction of urethral strictures and until several decades ago had been the most popular material for anterior urethroplasty [28,29,30]. However, penile skin has gradually been replaced by buccal mucosa grafts (BMGs) because of their advantageous histological properties and superior handling characteristics [12, 31, 32]. It has been shown that there was a much higher morbidity with penile skin flaps than with BMGs—including penile hematoma , skin necrosis, and fistula formation—and that the flap procedure was also technically more complex and less likely preferred by patients [33]. In addition, tunneling a skin flap through the scrotum is required in bulbar urethroplasty, which may tether the penis during erection [34]. Although penile skin flaps still have an important role in the armamentarium in anterior urethroplasty, their use in bulbar urethroplasty has become limited to exceptional cases such as long segmental strictures [35]. Penile skin has also been the mainstay of grafting material until buccal mucosal grafts became popularized. Compared with the preparation of a penile skin flap, harvesting a penile skin graft is technically simple and has lower complication rates [36]. However, recent studies show substitution urethroplasty using penile skin graft is inferior to that of using BMG. Lumen et al. performed a meta-analysis comparing outcomes of penile skin graft urethroplasty and BMG urethroplasty and found a slightly worse outcome in the penile skin graft group: 81.8% success versus 85.9% (p = 0.01) [37]. Barbagli also reported the long-term success rate of 359 patients who underwent one-stage anterior substitution urethroplasty with a minimum of 6 years follow-up. At 6 years oral mucosa (including buccal mucosa) showed a greater failure-free survival rate (78.0%) than penile skin (62%) and the only significant predictor of successful urethroplasty was the substitute tissue type [22]. Granieri et al. reported that changing the routine use of penile skin for augmented anastomotic urethroplasty to the routine use of buccal mucosa improved the recurrence rate (from 21.6% with penile skin to 5.8% with buccal mucosa) [32]. In addition, penile skin has to be avoided in the reconstruction of anterior urethral stricture associated with LS because it may become diseased and associated with a very high long-term failure rate [38]. Based on this, BMG has become the best urethral substitute among various tissues.
El-Kassaby first reported experience with adults undergoing urethroplasty (eight patients with bulbar urethral strictures) using BMG in 1993 [39]. Morey and McAninch thereafter showed a new technique for harvesting buccal mucosa and its use was popularized [40]. Buccal mucosa has several unique characteristics. The environment of the mouth ensures that buccal mucosa is highly resistant to infection and trauma. The epithelial layer is thick, making the graft easy to harvest and handle. It takes readily and inoculates early because it has a thin lamina propria and dense panlaminar vascular plexus. It also has a privileged immunology, and preclinical work suggests that it shows fibroblast behavior resulting in less fibrosis than skin and a profile quite different from that of skin. Buccal mucosa is an appropriate urethral substitute for strictures associated with LS [38]. Oral mucosa graft can be harvested not only from the inside of a cheek but also from the lower side of the tongue (lingual mucosa) or the inside of the lip (labial mucosa). Although lingual mucosa has characteristics similar to those of buccal mucosa and appears to be as efficacious as buccal mucosa [26], a lingual mucosa graft is typically not as wide as a buccal mucosa graft and is flimsier [34]. Lingual mucosa might therefore be better to use in patients with a short stricture requiring only a small amount of grafts, in patients with a lengthy stricture requiring additional grafts with a tissue other than buccal mucosa, and in patients for salvage urethroplasty whose buccal mucosa has already been harvested bilaterally. Labial mucosa can be managed in a similar way but is thinner and more difficult to handle. In addition, lip contracture is a devastating complication reported in 3–5% of patients after lip graft harvest [41,42,43]. The risk of numbness and deformity after lip grafts harvest is also high, and it is best to avoid use of labial mucosa grafts when possible.
Techniques used for harvesting buccal mucosa grafts are described elsewhere [44]. Nasal intubation is not mandatory but it is helpful for surgeons unfamiliar with this technique. The graft is designed in an ovoid shape for one-stage urethroplasty to adjust to the shape of the urethral opening or is designed in a rectangular shape for staged urethroplasty requiring a larger graft. It has to be kept in mind to harvest the graft about 1.5 cm from the parotid duct (Stensen duct) to prevent injury [44]. Another concern is whether or not the wound at the donor site has to be closed. Some studies have suggested that the incidence of postoperative complications in patients who undergo BMG harvesting is mainly influenced by the closure vs. non-closure of the donor site [43, 45]. Barbagli reported a survey of 553 patients revealing that 53.2% of them experienced no postoperative pain and 98.2% would undergo the surgery again. They concluded that harvesting from a single cheek with closure of the donor site was a safe procedure with high patient satisfaction [44]. Soave and colleagues recently conducted a randomized trial to find out whether or not closure of the donor site after harvesting buccal mucosa is necessary and found that non-closure of the resulting mucosal defect is not inferior to closure in terms of the intensity and nature of the consequent pain experienced by patients [46]. Ultimately, the decision to close the donor site primarily or leave it open is at the discretion of the surgeon.
Although buccal mucosa is a convenient source of donor tissue with many characteristics of the ideal urethral graft, harvesting BMG is associated with donor site complications [47]. To overcome the risk of comorbidity, Barbagli et al. developed tissue-engineered oral mucosa graft from tiny oral mucosa biopsy samples taken from the cheek (MukoCell® graft) and used it in one-stage substitution urethroplasty in 38 patients with anterior urethral stricture (three penile, 29 bulbar, and six penobulbar strictures) with a median stricture length of 5 cm, demonstrating with a median follow-up of 55 months a success rate comparable with that of conventional substitution oral mucosa urethroplasty [48]. Tissue-engineered graft is an emerging frontier of urethral reconstruction and holds substantial promise.
3 Preoperative Evaluation and Management
Accurate determination of the characteristics of a stricture is the first step in a successful urethroplasty. Prior to the examination, it is necessary to stabilize the stricture by “urethral rest”, especially in patients with a history of repeated urethral manipulations. “Urethral rest” is the concept of avoiding manipulation of the urethra for at least 3 months and allowing the urethral scar to mature before definitive urethroplasty [49]. For those with a high level of concern for urinary retention (rapid symptom recurrence after prior urethral dilation and/or increased difficulty with self-dilation), suprapubic tube (SPT) placement as soon as possible is recommended. Urethral rest promotes identification of severely fibrotic segments and facilitates proper selection of the urethroplasty technique [49]. A period of urethral rest resulted in more frequent stricture obliteration and about half of the cases was associated with a change in the planned operation approach [50].
A retrograde urethrogram (RUG) is the standard investigation of anterior urethral strictures, and a consecutive ascending and voiding cystourethrogram should also be carried out in order to better define the proximal limit of the stricture and assess the external sphincter complex and bladder neck [51]. However, these radiographic studies often underestimate stricture length because they are performed in an oblique position in relation to the anteroposterior x-ray beam, resulting in a shorter projected view of the stricture [52]. For very tight strictures , chronic high-pressure voiding dilates the proximal segment and makes the stricture appear to be shorter than it really is. Urethral surgeons should keep in mind that a RUG’s ability to accurately reveal stricture length and location and the extent of periurethral disease is limited. Endoscopy may be useful for visualizing the mucosa around the stricture. Pink mucosa is healthy but pale grey mucosa is a sign of submucosal fibrosis that needs to be reconstructed. This evaluation may be particular important in patients with previous urethroplasty or those managed with urethrotomy or dilation. Urethral ultrasound can be used as an adjunct to RUG and may be more sensitive than RUG in the assessment of stricture length and the degree of spongiofibrosis [53], but the clinical relevance of this possibly higher sensitivity is unclear [51].
The approach to bulbar urethral stricture repair is usually guided by the stricture’s appearance on retrograde urethrography, but intraoperative findings dictate the final operative plan. In all cases, intraoperative assessment and localization are the final determinants of how a stricture is reconstructed because they yield the most information [54].
4 Urethroplasty Categories and Selection of Urethroplasty Type
Stricture characteristics that influence the type of reconstruction include stricture length, anatomic position, degree of spongiofibrosis, etiology, and previous treatment [8, 12, 38] (Fig. 16.1). The bulbar urethra lies between the penoscrotal junction and membranous urethra and is subdivided into two parts, proximal and distal. The proximal bulbar urethra is defined as the segment within 5 cm of the membranous urethra and the distal urethra is defined as the adjoining segment extending to the penoscrotal junction [18]. The bulbar urethra is surrounded by the thickest portion of the corpus spongiosum and is eccentrically placed toward the dorsum. Thus the surrounding tissues of the corpus spongiosum are dorsally thin and ventrally thick [12]. The reconstructive methods used in the distal and proximal bulbar urethra differ markedly because of the different thicknesses and elasticities of the associated spongiosum tissue.
Surgical procedures of substitution urethroplasty are categorized into one-stage and staged procedures and almost all bulbar strictures can be repaired by one-stage urethroplasty. There are three potential options for a one-stage procedure: onlay augmentation (incise the stricture and carry out a patch augmentation), augmented anastomosis (excise the stricture and restore a roof or floor strip of native urethra augmented by a patch), and tube augmentation (excise the stricture and put in a circumferential patch) [12]. One-stage tube augmentation has a high risk of stricture recurrence regardless of the stricture location and should be avoided [12, 38]. Onlay graft urethroplasty is the method used widely to repair bulbar urethral strictures. Ventral and dorsal onlay urethroplasties are the most popular types of substitution urethroplasty, and the choice of ventral or dorsal remains a matter of debate [12, 55]. Two other options are a dorsal inlay with ventral sagittal urethrotomy without urethral dissection [56] and a lateral onlay procedure with only one-sided urethral dissection [57, 58]. To date, there is no difference in success rates among the grafting positions [12, 55, 59,60,61,62,63]. When a bulbar urethral stricture has an obliterative or nearly obliterative segment, what should be used is a combination of EPA and onlay augmentation called augmented anastomotic urethroplasty [64], and an onlay augmentation using two grafts applied both dorsally and ventrally to obtain an adequate lumen without transecting the urethra (two-sided dorsal plus ventral onlay urethroplasty) [65,66,67].
Bulbar urethroplasties are usually performed in a social lithotomy position with a midline perineal incision or lambda incision, depending on the surgeon’s preference. Substitution bulbar urethroplasty using BMG is discussed below.
4.1 Ventral Onlay
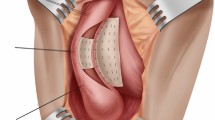
Ventral onlay for bulbar urethral strictures was first described by Morey and McAninch in 1996 [68]. Once the stricture is located, a midline sagittal incision is made through the ventral spongiosum, leaving the surrounding supportive structure intact. The graft is then sewn directly to the edges of the urethrotomy to augment the urethral lumen, and then the corpus spongiosum is closed over the graft to provide a well-vascularized bed (Fig. 16.2). The advantages of the ventral onlay procedure include limited urethral mobilization with preservation of perforating arteries and shorter operative time and easier technique compared to the dorsal onlay procedure. In obese patients and patients with proximal bulbar stricture, dorsal graft placements are typically more difficult to sew in a deep and proximal wound. In patients who have undergone repeated DVIUs or urethroplasties, the urethral lumen may be adherent and firmly fixed to the tunica albuginea and urethral mobilization from the corpora cavernosa is difficult. In these patients the use of a ventral onlay procedure is preferable. Ventral onlay urethroplasty, however, is usually performed only for midbulbar and proximal bulbar strictures because only in those areas is the bulb wide enough for adequate spongioplasty (Fig. 16.1). Furthermore, ventral onlay is not suitable for traumatic bulbar urethral strictures because a severely scarred corpus spongiosum does not work as a vascularized graft bed. Because the ventral spongiosum is more robust it bleeds vigorously, compromising visualization and making graft placement challenging. Although ventral spongiosum tissue is thick and its blood supply is rich, it is not particularly firm compared with the strength of the underlying corporal bodies. This fragility of the ventral spongiosum may increase the risk of graft sacculation.
Ventral onlay. (a) The corpus spongiosum is opened along its ventral surface and (b) the urethral lumen is fully exposed, extending the urethrotomy distally and proximally to the stenosis. (c) The graft is sutured to the edge of the urethral mucosa plate. (d) The spongiosum tissue is closed over the graft. (e) Schematic image of ventral onlay
Regarding surgical outcome , a recent review including 563 patients in 24 studies reported that with an average follow-up of 34.3 months the overall average success rate of ventral onlay bulbar urethroplasty was 88.8% [55]. BMG was used as substitute urethra in most of the patients evaluated in that review. Barbagli et al. reported that in a retrospective study of 214 patients with bulbar stricture (median stricture length 4.4 cm) who underwent ventral onlay urethroplasty using only BMG in a single high-volume center , with a median follow-up of 54 months the success rate was 85.5% [69].
4.2 Dorsal Onlay
The dorsal onlay urethroplasty pioneered by Barbagli [70] involves mobilizing the bulbar urethra and rotating it 180 ° so the urethrotomy can be made on the dorsal aspect of the strictured segment. A graft is then quilted to the underlying tunica albuginea of the corpora cavernosa both along the edges of the graft and across the face of the graft, which helps prevent graft contracture and graft elevation due to an underlying hematoma. The dorsally fixed buccal graft is then sutured to both edges of the urethrotomy with the urethra rotated back to its original position to cover the grafted area (Fig. 16.3). Among the advantages of the dorsal onlay procedure is a more stable and reliably well-vascularized graft bed and less possibility of sacculation [60]. The corpus spongiosum in the bulbar urethra is thicker ventrally and thinner dorsally. Furthermore, the urethral lumen is located dorsally rather than centrally; thus, a dorsal incision may be more likely to preserve the residual blood supply to the spongiosum tissue and to result in less intraoperative blood loss and less possibility of impairing visualization during urethral exposure and graft placement. The dorsal approach to graft placement may also be preferred because it is the most versatile irrespective of the location of the corpus spongiosum and has been successfully used in all parts of the bulbar urethra (Fig. 16.1). The dorsal onlay procedure is preferable in the distal bulbar urethra because the bulb is not wide enough for a spongioplasty adequate for ventral onlay and attaching the graft to the tunica albuginea of the corpora cavernosa is easier in the distal bulbar urethra. Another advantage of the dorsal onlay procedure is its excellent adaptability to other procedures. When complete or nearly complete obliteration of the urethra makes a single augmentation graft impossible, one can enhance the dorsal graft with either an augmented anastomotic reconstruction [64] or a combination of ventral inlay graft [71]. Disadvantages of the dorsal onlay procedure are that circumferential mobilization disrupts the surrounding microvasculature and visualization of the proximal urethra is challenging.
Dorsal onlay . (a) The bulbar urethral is circumferentially mobilized and rotated. (b) The dorsal urethral surface is incised along the midline to expose the entire stricture. (c) The graft is spread, fixed over the corpora cavernosa. (d) The bulbar urethra is rotated back and the margin of the graft is sutured to the margin of the urethral mucosal plate. (e) Schematic image of dorsal onlay
A recent review described the success rate of 35 studies of the dorsal onlay bulbar urethroplasty in a total of 934 patients. The average success rate, with an average follow-up of 42.2 months, was 88.4%, which is almost the same as the success rate of ventral onlay bulbar urethroplasty [55]. Subsequent retrospective analysis also showed similar success rates of dorsal onlay and ventral onlay procedures for bulbar urethral stricture [60,61,62]. A prospective randomized study comparing dorsal onlay and ventral onlay procedures in BMG urethroplasty for bulbar stricture showed comparable results (92.5% for dorsal onlay and 90% for ventral onlay) [63].
4.3 Lateral Onlay
The lateral graft placement described by Barbagli is an amalgam of the ventral and dorsal approaches [57]. Kulkarni et al. subsequently proposed a one-sided dorsolateral graft urethroplasty that, eliminating the need for full circumferential mobilization of the urethra and preserving the lateral vascular and nerve supply to the urethra and establishing secure dorsal grafting, is a minimally invasive alternative to conventional dorsal onlay urethroplasty [58]. The urethra is dissected from the corpora cavernosa only along one side, starting from the distal tract where bulbospongiosus muscles are absent, and is partially rotated leaving the other side attached to the corpora cavernosa, and leaving the bulbospongiosum muscle and the central tendon of the perineum intact (Fig. 16.4). The distal extent of the stricture is identified, the dorsolateral urethral surface incised along the midline, and the urethral lumen exposed. The stricture is then incised along its entire length by extending the urethrotomy both distally and proximally into the healthy urethra. The buccal mucosa graft is spread fixed over the tunica albuginea and sutured to the margin of the urethral mucosa. Then the urethra is rotated over the graft back into its original position. Barbagli commented that the lateral opening of the urethral surface can avoid the pitfall of ventral and dorsal urethrotomy in patients with long strictures located in a full-sized bulbar urethra; that is, it enables one to avoid the erectile dysfunction and serious bleeding from thick spongiosum tissue that are caused by dissecting bulbar urethra from the corpora cavernosa [57].
Lateral onlay. (a) The urethra is mobilized from the albuginea only along the left side. (b) The dorsolateral side of the urethra is incised longitudinally. (c) The graft is sutured to the underlying albuginea, and the right margin of the graft is sutured to the left margin of the urethral plate (d) and on the other side. (e) Schematic image of lateral onlay
Barbagli et al. described buccal mucosa lateral onlay bulbar urethroplasty in six patients. The first reference was at 42 months follow-up, and the same six patients were later described at 77 months follow-up [57, 72]. The success rate remained at 83% over this follow-up period. Kulkarni et al. reported their experience in 12 bulbar urethral strictures with short follow-up (mean 22 months); their procedure had a success rate of 92% [58].
4.4 Dorsal inlay
Asopa et al. described in 2001 a dorsal inlay technique using a dorsal free graft to treat urethral stricture by the ventral sagittal urethrotomy approach without urethral mobilization (Fig. 16.5) [56]. The urethra is slit open ventrally and the stricture is laid open. The full thickness of the dorsal urethra is then incised in the midline, providing an adequate elliptical raw area between the incised dorsal edges of the urethra. Grafts are placed over the raw area of the incised dorsal urethra (tunica albuginea of corporal body) and sutured to the edge of the incised dorsal urethra, making it possible to obtain an adequate urethral lumen width. The main advantage of the dorsal inlay is that the grafting space is created without circumferential mobilization of the urethra, thus preserving the circumflex and perforating arteries to the spongiosum. Additionally, proximal exposure of the stricture is easier than it is with the dorsal approach. However, compared with the dorsal onlay approach, the dorsal inlay graft contributes less to increasing the urethral caliber. The limited mobilization results in a relatively smaller potential graft width, which may limit the applicability of this approach to narrow strictures. Additionally, with the dorsal inlay graft there is more than one urethrotomy , potentially increasing the possibility of blood loss, urethral stricture, or fistula formation.
Dorsal inlay. (a) The urethra is left adherent to the corpora cavernosa and is longitudinally opened ventrally extending to the distal and proximal healthy urethra. (b) The strictured urethral plate is longitudinally opened dorsally. (c) The graft is sutured to the margins of the urethral plate and fixed to the corpora cavernosa. (d) The urethra is retubularized over a urethral catheter. (e) Schematic image of dorsal inlay
Pisapati reported the results of 58 patients who underwent bulbar urethroplasty with the dorsal inlay technique and the overall success rate at a mean follow-up of 42 months was 87% [73]. Aldaqadossi et al. conducted a prospective randomized study comparing the success rates of dorsal inlay and dorsal onlay BMG urethroplasties for 47 anterior urethral strictures and showed that they provide similar success rates (88% for dorsal onlay and 86.4% for dorsal inlay) [59]. Compared with dorsal onlay, dorsal inlay was shown to have a shorter operative time and less blood loss [59]. However, the main drawback of this technique is that the urethral plate needs to be more than 1 cm wide, and the graft that can be inlayed is typically narrower than the wide grafts that can be achieved with an onlay procedure [34].
4.5 Augmented Anastomotic Urethroplasty
Traumatic strictures and redo surgery with extensive scar tissue in the corpus spongiosum are two main indications for EPA in bulbar urethral strictures. As mentioned above, EPA provides for the most successful outcome in bulbar urethral reconstruction. However, there are times when, following stricture excision and urethral spatulation, too much tension would be present for a primary anastomosis. Excising strictured segments and making an anastomosis without tension is impossible, so using EPA for a long bulbar urethral stricture causes chordee. Nor can an adequate urethral lumen be achieved by an onlay augmentation on a single side. In 2001, Guralnick and Webster introduced the term “augmented anastomosis” to describe a combination of the two conventional approaches of excision with an onlay technique for bulbar strictures not amenable to either EPA or onlay augmentation [64]. In brief, the most obliterated segment of the stricture is excised, and stricturotomy is performed into the healthy urethra proximally and distally. Either the ventral or dorsal wall is then anastomosed and the remaining defect in the other side is repaired with a graft [64] (Fig. 16.6). With this technique, up to 2 or 3 cm of strictured urethra may be excised, followed by reapproximation of either the dorsal or ventral walls.
Augmented anastomotic urethroplasty. (a) The bulbar urethra is circumferentially mobilized and a tight stricture is completely removed. (b) Both proximal and distal urethral walls are dorsally spatulated (c) and sutured to the graft fixed over the corpora cavernosa. (d) The ventral side of the urethra is then closed. (e) Schematic image of augmented anastomotic urethroplasty
A series of 29 patients underwent an augmented anastomotic urethroplasty for bulbar stricture (mean length 1.5 cm) in which the ventral urethral strip was anastomosed and the dorsal defect augmented with a buccal mucosa graft. In that series the stricture-free rate at a mean follow-up of 28 months was 93% [64]. Hoy et al. reported the outcome of 163 patients who underwent the same procedure for longer bulbar strictures (mean 4.5 cm) and also showed excellent results: at the median follow-up of 31 months, 96.9% had no stricture recurrence [74]. This high success rate could be due to complete excision of the narrowest stricture segment, which has the greatest risk of recurrence [74]. They also suggested that strictures longer than 5 cm are suitable to repair using this technique but can be associated with slightly greater recurrence rates. El-Kassaby et al. reported the outcome of augmented anastomotic urethroplasty using ventral onlay buccal mucosal patch grafts in 234 patients with long bulbar urethral strictures (mean 4.2 cm) and that with the mean follow-up of 36 months the overall success rate was 93.7% [75]. As in onlay augmentation, the different graft positions in augmented anastomotic urethroplasty have shown no difference in success rate.
4.6 Two-Sided Dorsal Plus Ventral Double Graft
Ideally, tight stricture segments should be removed, but transection procedures can impair sexual function as a consequence of the vascular damage and urethral shortening [14, 16, 19]. The use of augmented anastomotic urethroplasty is limited by stricture length, with the longest stricture treated with it in one prominent series being only 2 cm [64]. An EPA or augmented anastomotic urethroplasty may not be feasible in patients with longer obliterative segments. For these kinds of patients, a solution is a combined two-sided dorsal plus ventral double grafting. Two-sided dorsal plus ventral double grafting can be performed in two ways. One is a combination of ventral onlay with dorsal inlay technique described by Palminteri et al. [67], and the other is a combination of dorsal onlay with ventral inlay described by Gelman et al. [65]. In the former technique the stricture is opened ventrally without mobilizing the bulbar urethra and the exposed urethral plate is incised in the midline and augmented dorsally and ventrally using two oral grafts (Fig. 16.7a, b) [67]. Palminteri et al. reported a single-center experience with 166 patients who underwent a combination of dorsal inlay and ventral onlay technique for tight bulbar strictures: at a median follow-up of 47 months, 149 of the patients’ repairs (89.8%) were successful [66]. In the latter technique the mobilized corpus spongiosum is incised dorsally without transecting it, thereby preserving the continuity of the blood supply within the spongy tissue and also quilting buccal mucosa both dorsally on corpus cavernosa and ventrally on the spongiosum tissue (Fig. 16.7) [65]. Gelman et al. reported a series of 18 patients who underwent bulbar urethroplasty with two-sided double grafting combining dorsal onlay and ventral inlay techniques: with a mean follow-up of 50 months the success rate was 94% [65].
5 Postoperative Care
The incisions are dressed with hydrocolloid dressing followed by fluff gauze. A scrotal supporter is used to hold the dressing in place and to ensure gentle compression and immobilization that reduces edema without compromising blood supply. The perineum and cheek (donor site) are cooled with ice bags. Oral intake is started on the first postoperative day with liquid meals. To reduce donor site pain and promote wound healing, we advise patients to gargle with saline containing sodium gualenate hydrate and lidocaine according to the level of pain. Whatever types are used, grafts rely on a healthy and well-vascularized recipient site for survival, and the initial blood supply via passive diffusion (imbibition) is gradually replaced by inosculation over the first 48 hours as host capillaries grow through the graft [76]. Therefore, it is important to keep a patient’s mobility limited during the first 48 hours postoperative for secure fixation of the graft to the host bed. A 14–16 Fr 100% silicone catheter used as a stent and to divert the urine for two to three weeks is removed when a peri-catheter urethrogram shows no extravasation. Because most patients with urethral stricture have a significant post-void residual urine volume, perioperative antibiotics should be administrated according to the results of urine culture and bacterial sensitivity checked prior to surgery until the patient’s urethral catheter is removed. Even with antibiotics the failure rate is twice as high when the preoperative urine is infected [77].
There is no established follow-up strategy after urethroplasty. In our practice, patients are followed up at 3, 6, and 12 months and annually thereafter by uroflowmetry and questionnaires using validated patient reported outcome measures (PROMs) [78]. Flexible cystoscopy is done at the sixth postoperative month and whenever a recurrent stricture is suspected on uroflowmetry or from a PROM.
6 Conclusions
Substitution urethroplasty is the procedure of choice for a long stricture in the proximal bulbar urethra or a stricture of any length located anywhere from the distal bulbar urethra and for strictures in patients who worry about sexual dysfunction. BMG has become the best urethral substitute among various tissues. The key to success is to select an appropriate procedure according to the status and location of the stricture.
Key Summary Points
-
Open surgical repair using free grafts or pedicled skin flaps, called substitution urethroplasty, is the gold standard procedure for bulbar urethral strictures not amenable to excision and primary anastomosis.
-
Buccal mucosa harvested from inner cheek is an ideal urethral substitute and is the tissue most often used for substitution urethroplasty because of its compatibility with urethral tissue and the ease of harvesting and handling it.
-
Most bulbar urethral strictures can be repaired by one-stage urethroplasty, and buccal mucosa graft onlay augmentation is the most preferred procedure.
-
Onlay augmentation on the ventral side (ventral onlay) or dorsal side (dorsal onlay) has been widely used, and other options are dorsal inlay with ventral sagittal urethrotomy or lateral onlay with one-sided urethral dissection. Regarding the association of the graft position and the success rate, there is no significant difference in surgical outcome among the different graft positions.
-
In bulbar urethral strictures with obliterative or nearly obliterative segments, either a two-sided dorsal plus ventral onlay augmentation or a combination of excision and primary anastomosis and onlay augmentation (augmented anastomotic urethroplasty) is the procedure of choice.
References
Alwaal A, Blaschko SD, McAninch JW, et al. Epidemiology of urethral strictures. Transl Androl Urol. 2014;3:209.
Latini JM, McAninch JW, Brandes SB, et al. SIU/ICUD consultation on urethral strictures: epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology. 2014;83:S1.
Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107:6.
Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol. 2009;182:983.
Palminteri E, Berdondini E, Verze P, et al. Contemporary urethral stricture characteristics in the developed world. Urology. 2013;81:191.
Liu JS, Hofer MD, Oberlin DT, et al. Practice patterns in the treatment of urethral stricture among American urologists: a paradigm change? Urology. 2015;86:830.
Buckley JC, Heyns C, Gilling P, et al. SIU/ICUD consultation on urethral strictures: dilation, internal urethrotomy, and stenting of male anterior urethral strictures. Urology. 2014;83:S18.
Morey AF, Watkin N, Shenfeld O, et al. SIU/ICUD consultation on urethral strictures: anterior urethra – primary anastomosis. Urology. 2014;83:S23.
Andrich DE, Mundy AR. Non-transecting anastomotic bulbar urethroplasty: a preliminary report. BJU Int. 2012;109:1090.
Jordan GH, Eltahawy EA, Virasoro R. The technique of vessel sparing excision and primary anastomosis for proximal bulbous urethral reconstruction. J Urol. 2007;177:1799.
Lumen N, Poelaert F, Oosterlinck W, et al. Nontransecting anastomotic repair in urethral reconstruction: surgical and functional outcomes. J Urol. 2016;196:1679.
Chapple C, Andrich D, Atala A, et al. SIU/ICUD consultation on urethral strictures: the management of anterior urethral stricture disease using substitution urethroplasty. Urology. 2014;83:S31.
Andrich DE, Dunglison N, Greenwell TJ, et al. The long-term results of urethroplasty. J Urol. 2003;170:90.
Eltahawy EA, Virasoro R, Schlossberg SM, et al. Long-term followup for excision and primary anastomosis for anterior urethral strictures. J Urol. 2007;177:1803.
Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: analysis of 168 patients. J Urol. 2002;167:1715.
Morey AF, Kizer WS. Proximal bulbar urethroplasty via extended anastomotic approach – what are the limits? J Urol. 2006;175:2145.
Mundy AR. Anastomotic urethroplasty. BJU Int. 2005;96:921.
Terlecki RP, Steele MC, Valadez C, et al. Grafts are unnecessary for proximal bulbar reconstruction. J Urol. 2010;184:2395.
Barbagli G, De Angelis M, Romano G, et al. Long-term followup of bulbar end-to-end anastomosis: a retrospective analysis of 153 patients in a single center experience. J Urol. 2007;178:2470.
Palminteri E, Berdondini E, De Nunzio C, et al. The impact of ventral oral graft bulbar urethroplasty on sexual life. Urology. 2013;81:891.
Santucci RA. Substitution urethroplasty or anastomotic urethroplasty for bulbar urethra strictures? Or endoscopic urethrotomy? Opinion: substitution urethroplasty. Int Braz J Urol. 2015;41:613.
Barbagli G, Kulkarni SB, Fossati N, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol. 2014;192:808.
Liu JS, Han J, Said M, et al. Long-term outcomes of urethroplasty with abdominal wall skin grafts. Urology. 2015;85:258.
Ozgok Y, Ozgur Tan M, Kilciler M, et al. Use of bladder mucosal graft for urethral reconstruction. Int J Urol. 2000;7:355.
Palminteri E, Berdondini E, Fusco F, et al. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology. 2012;79:695.
Simonato A, Gregori A, Ambruosi C, et al. Lingual mucosal graft urethroplasty for anterior urethral reconstruction. Eur Urol. 2008;54:79.
Warner JN, Malkawi I, Dhradkeh M, et al. A multi-institutional evaluation of the management and outcomes of long-segment urethral strictures. Urology. 2015;85:1483.
McAninch JW. Reconstruction of extensive urethral strictures: circular fasciocutaneous penile flap. J Urol. 1993;149:488.
Morey AF, Tran LK, Zinman LM. Q-flap reconstruction of panurethral strictures. BJU Int. 2000;86:1039.
Orandi A. One-stage urethroplasty. Br J Urol. 1968;40:717.
Barbagli G, Balo S, Montorsi F, et al. History and evolution of the use of oral mucosa for urethral reconstruction. Asian J Urol. 2017;4:96.
Granieri MA, Webster GD, Peterson AC. The evolution of urethroplasty for bulbar urethral stricture disease: lessons learned from a single center experience. J Urol. 2014;192:1468.
Dubey D, Vijjan V, Kapoor R, et al. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol. 2007;178:2466.
Brandes S, Morey A. Advanced urethral and genital reconstructive surgery: New York: Humana Press; 2013.
Morey AF. Re: the use of penile skin graft versus penile skin flap in the repair of long bulbo-penile urethral stricture: a prospective randomized study. J Urol. 2011;186:2298.
Dubey D, Kumar A, Bansal P, et al. Substitution urethroplasty for anterior urethral strictures: a critical appraisal of various techniques. BJU Int. 2003;91:215.
Lumen N, Oosterlinck W, Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int. 2012;89:387.
Wessells H, Angermeier KW, Elliott S, et al. Male urethral stricture: AUA guideline. J Urol. 2016;197:182.
el-Kasaby AW, Fath-Alla M, Noweir AM, et al. The use of buccal mucosa patch graft in the management of anterior urethral strictures. J Urol. 1993;149:276.
Morey AF, McAninch JW. Technique of harvesting buccal mucosa for urethral reconstruction. J Urol. 1996;155:1696.
Fichtner J, Filipas D, Fisch M, et al. Long-term outcome of ventral buccal mucosa onlay graft urethroplasty for urethral stricture repair. Urology. 2004;64:648.
Meneghini A, Cacciola A, Cavarretta L, et al. Bulbar urethral stricture repair with buccal mucosa graft urethroplasty. Eur Urol. 2001;39:264.
Wood DN, Allen SE, Andrich DE, et al. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol. 2004;172:580.
Barbagli G, Fossati N, Sansalone S, et al. Prediction of early and late complications after oral mucosal graft harvesting: multivariable analysis from a cohort of 553 consecutive patients. J Urol. 2014;191:688.
Rourke K, McKinny S, St Martin B. Effect of wound closure on buccal mucosal graft harvest site morbidity: results of a randomized prospective trial. Urology. 2012;79:443.
Soave A, Dahlem R, Pinnschmidt HO, et al. Substitution urethroplasty with closure versus nonclosure of the buccal mucosa graft harvest site: a randomized controlled trial with a detailed analysis of oral pain and morbidity. Eur Urol. 2018;73:910.
Barbagli G, Lazzeri M. Re: tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Eur Urol. 2011;60:1303.
Barbagli G, Akbarov I, Heidenreich A, et al. Anterior urethroplasty using a new tissue engineered oral mucosa graft: surgical techniques and outcomes. J Urol. 2018;200:448.
Terlecki RP, Steele MC, Valadez C, et al. Urethral rest: role and rationale in preparation for anterior urethroplasty. Urology. 2011;77:1477.
Moncrief T, Gor R, Goldfarb RA, et al. Urethral rest with suprapubic cystostomy for obliterative or nearly obliterative urethral strictures: urethrographic changes and implications for management. J Urol. 2018;199:1289.
Angermeier KW, Rourke KF, Dubey D, et al. SIU/ICUD consultation on urethral strictures: evaluation and follow-up. Urology. 2014;83:S8.
Gupta N, Dubey D, Mandhani A, et al. Urethral stricture assessment: a prospective study evaluating urethral ultrasonography and conventional radiological studies. BJU Int. 2006;98:149.
Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. J Urol. 2000;163:1070.
Kuo TL, Venugopal S, Inman RD, et al. Surgical tips and tricks during urethroplasty for bulbar urethral strictures focusing on accurate localisation of the stricture: results from a tertiary centre. Eur Urol. 2015;67:764.
Mangera A, Patterson JM, Chapple CR. A systematic review of graft augmentation urethroplasty techniques for the treatment of anterior urethral strictures. Eur Urol. 2011;59:797.
Asopa HS, Garg M, Singhal GG, et al. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology. 2001;58:657.
Barbagli G, Palminteri E, Guazzoni G, et al. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005;174:955.
Kulkarni S, Barbagli G, Sansalone S, et al. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int. 2009;104:1150.
Aldaqadossi H, El Gamal S, El-Nadey M, et al. Dorsal onlay (Barbagli technique) versus dorsal inlay (Asopa technique) buccal mucosal graft urethroplasty for anterior urethral stricture: a prospective randomized study. Int J Urol. 2014;21:185.
Figler BD, Malaeb BS, Dy GW, et al. Impact of graft position on failure of single-stage bulbar urethroplasties with buccal mucosa graft. Urology. 2013;82:1166.
Hosseini J, Kaviani A, Hosseini M, et al. Dorsal versus ventral oral mucosal graft urethroplasty. Urol J. 2011;8:48.
Pathak HR, Jain TP, Bhujbal SA, et al. Does site of buccal mucosa graft for bulbar urethra stricture affect outcome? A comparative analysis of ventral, dorso-lateral and dorsal buccal mucosa graft augmentation urethroplasty. Turk J Urol. 2017;43:350.
Vasudeva P, Nanda B, Kumar A, et al. Dorsal versus ventral onlay buccal mucosal graft urethroplasty for long-segment bulbar urethral stricture: a prospective randomized study. Int J Urol. 2015;22:967.
Guralnick ML, Webster GD. The augmented anastomotic urethroplasty: indications and outcome in 29 patients. J Urol. 2001;165:1496.
Gelman J, Siegel JA. Ventral and dorsal buccal grafting for 1-stage repair of complex anterior urethral strictures. Urology. 2014;83:1418.
Palminteri E, Lumen N, Berdondini E, et al. Two-sided dorsal plus ventral oral graft bulbar urethroplasty: long-term results and predictive factors. Urology. 2015;85:942.
Palminteri E, Manzoni G, Berdondini E, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol. 2008;53:81.
Morey AF, McAninch JW. When and how to use buccal mucosal grafts in adult bulbar urethroplasty. Urology. 1996;48:194.
Barbagli G, Montorsi F, Guazzoni G, et al. Ventral oral mucosal onlay graft urethroplasty in nontraumatic bulbar urethral strictures: surgical technique and multivariable analysis of results in 214 patients. Eur Urol. 2013;64:440.
Barbagli G, Selli C, Tosto A, et al. Dorsal free graft urethroplasty. J Urol. 1996;155:123.
Gelman J, Sohn W. 1-stage repair of obliterative distal urethral strictures with buccal graft urethral plate reconstruction and simultaneous onlay penile skin flap. J Urol. 2011;186:935.
Barbagli G, Guazzoni G, Lazzeri M. One-stage bulbar urethroplasty: retrospective analysis of the results in 375 patients. Eur Urol. 2008;53:828.
Pisapati VL, Paturi S, Bethu S, et al. Dorsal buccal mucosal graft urethroplasty for anterior urethral stricture by Asopa technique. Eur Urol. 2009;56:201.
Hoy NY, Kinnaird A, Rourke KF. Expanded use of a dorsal onlay augmented anastomotic urethroplasty with buccal mucosa for long segment bulbar urethral strictures: analysis of outcomes and complications. Urology. 2013;81:1357.
El-Kassaby AW, El-Zayat TM, Azazy S, et al. One-stage repair of long bulbar urethral strictures using augmented Russell dorsal strip anastomosis: outcome of 234 cases. Eur Urol. 2008;53:420.
Bryk DJ, Yamaguchi Y, Zhao LC. Tissue transfer techniques in reconstructive urology. Korean J Urol. 2015;56:478.
Roehrborn CG, McConnell JD. Analysis of factors contributing to success or failure of 1-stage urethroplasty for urethral stricture disease. J Urol. 1994;151:869.
Jackson MJ, Sciberras J, Mangera A, et al. Defining a patient-reported outcome measure for urethral stricture surgery. Eur Urol. 2011;60:60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Horiguchi, A., Shinchi, M. (2020). Substitution Urethroplasty for Bulbar Urethral Strictures. In: Martins, F.E., Kulkarni, S.B., Köhler, T.S. (eds) Textbook of Male Genitourethral Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-030-21447-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-21447-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21446-3
Online ISBN: 978-3-030-21447-0
eBook Packages: MedicineMedicine (R0)