Abstract
I consider this chapter to be one of the most important chapters in the book. We know that a drug has to form a solution in an aqueous medium if it will be administered IV. We also know that drugs can cross membrane barriers only as molecular dispersions. Therefore, they can get absorbed and distributed in the body only if they mix and dissolve in biological fluids. Drug dissolution and membrane crossing occur by diffusion. Through nice descriptions and activities related to the phenomenon of diffusion and osmosis, you will understand the process of dissolution and you will relate it to drug absorption and distribution into tissues. You will correlate the physicochemical properties of drugs to their ability to cross cell plasma membranes and will understand the effect of food and gastrointestinal motility on drug absorption.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Reference
M.L. Raff, Principles of Physical Chemistry, 1st edn. (Prentice Hall, Upper Saddle River, 2001)
Author information
Authors and Affiliations
8.1 Electronic Supplementary Material
(MP4 571431 kb)
(MP4 1114284 kb)
(MP4 400053 kb)
Exercises
Exercises
-
8.1.
Which of the following molecules are anticipated to form a solution in water? Choose all that apply.
-
(a)
Nonionic polar
-
(b)
Nonionic apolar
-
(c)
Ionic
-
(d)
Ionic surfactant
-
(e)
Nonionic surfactant
-
(a)
-
8.2.
Which of the following statements are correct? Choose all that apply.
-
(a)
Molecules that show stronger cohesive than adhesive forces are most likely to dissolve in a solvent.
-
(b)
Formation of solute–solvent non-covalent bonds increases the energy of the system.
-
(c)
Formation of solute–solvent non-covalent bonds favors solution formation.
-
(d)
Molecules of very different physicochemical properties are most likely to be separated in a solution.
-
(e)
Water molecules form hydrogen bonds with solutes in solution.
-
(a)
-
8.3.
Choose the correct statement. An aqueous solution is at equilibrium when
-
(a)
Brownian motion of solutes stops
-
(b)
Random motion of solutes stops
-
(c)
Solute distribution in the solution is homogeneous
-
(d)
Solvent advection stops
-
(e)
The water concentration equals the solute concentration
-
(a)
-
8.4.
Given Eq. 8.1, if dm/dt, D, h, and A are expressed in units of g/s, m2/s, m, and m2, respectively, what are the units of concentration? Select all that apply.
-
(a)
g/L
-
(b)
mg/L
-
(c)
10−3 g/L
-
(d)
mg/m3
-
(e)
mol/m3
-
(a)
-
8.5.
Choose the statements that describe the process of diffusion. Select all that apply.
-
(a)
It is the movement of solute molecules from low water concentration to high water concentration.
-
(b)
It is the movement of water molecules due to osmosis from high to low water concentration.
-
(c)
It is the bulk movement of solute and solvent molecules from high to low solute concentration.
-
(d)
It is the movement of solutes from low to high temperature.
-
(e)
It is the movement of solutes with bulk flow down their concentration gradient.
-
(a)
-
8.6.
Write down the definition of diffusion, advection, convection, and osmosis before answering the next question.
-
8.7.
The movement of particles from a region of high to a region of low particle concentration without bulk motion is called:
-
(a)
Diffusion
-
(b)
Osmosis
-
(c)
Convection
-
(d)
Advection
-
(e)
Tonicity
-
(a)
-
8.8.
Which of the following are not properties of passive diffusion?
-
(a)
Diffusion is unidirectional.
-
(b)
Diffusion always proceeds down the concentration gradient.
-
(c)
The rate of diffusion (dm/dt) diminishes with increasing thickness of the membrane that separates the two solutions.
-
(d)
The rate of diffusion (dm/dt) increases with increasing concentration difference on the two sides of the membrane.
-
(e)
Diffusion has a very short length scale.
-
(f)
The diffusion coefficient of a molecule is higher in a gas phase than in a liquid phase/state.
-
(g)
The diffusivity of a molecule varies with temperature.
-
(h)
Lighter molecules diffuse faster than heavier molecules.
-
(i)
The diffusion coefficient of a solute molecule increases with the viscosity of the solvent.
(Answer: Lighter doesn’t mean smaller. All other explanations are in the book.)
-
(a)
-
8.9.
Which of the following is wrong about the terms of the diffusion equation shown below?
$$ \frac{\mathrm{d}m}{\mathrm{d}t}={P}_{\mathrm{m}}\bullet A\bullet \left({C}_1-{C}_2\right) $$-
(a)
A is the surface area of the membrane.
-
(b)
(C1 − C2) is the concentration gradient on the two sides of the membrane.
-
(c)
Pm is the solvent/membrane partition coefficient of the molecule.
-
(d)
m is the mass of the molecule that goes through the membrane.
-
(e)
dm/dt is the rate of mass transport of the molecule through the membrane.
-
(a)
-
8.10.
Which of the following is correct about the driving force for drug absorption via passive diffusion?
-
(a)
The driving force is the lipophilicity of the drug.
-
(b)
The driving force is the small size of the drug.
-
(c)
The driving force is the concentration gradient of the drug.
-
(d)
The driving force is the polarity of the drug.
-
(e)
The driving force is the presence of uptake transporters.
-
(a)
-
8.11.
Why protein-bound drugs cannot participate in the passive diffusion of drugs through membranes? Choose the correct statement.
-
(a)
Because protein-bound drugs are too hydrophilic.
-
(b)
Because protein-bound drugs are too polar.
-
(c)
Because protein-bound drugs are denatured.
-
(d)
Because protein-bound drugs are pharmacologically inactive.
-
(e)
Because the particle size of protein-bound drugs is too big to cross the membrane.
-
(a)
-
8.12.
Which of the following is not a factor that determines drug permeability across a membrane?
-
(a)
Drug polarity
-
(b)
Presence of uptake and efflux transporters in the membrane
-
(c)
Molecular weight of the drug
-
(d)
Temperature of the membrane
-
(e)
Drug hydrophobicity
-
(a)
-
8.13.
Which of the following low MW substances could diffuse through cell plasma membrane? Choose all that apply.
-
(a)
Nonionic polar
-
(b)
Nonionic apolar
-
(c)
Ionic
-
(d)
Ionic surfactant
-
(e)
Nonionic surfactant
-
(a)
-
8.14.
Which of the following does not affect the passive diffusion of a nonionic molecule through a biological membrane?
-
(a)
The hydrophobicity of the molecule
-
(b)
The molecular weight of the molecule
-
(c)
The particle size of the nonionic molecule
-
(d)
The pH of the drug solution
-
(e)
The permeability coefficient of the molecule
-
(a)
-
8.15.
Choose the correct statement regarding barrier permeability, not necessarily biological, from below.
-
(a)
The bigger the molecule, the higher its permeability.
-
(b)
Permeability depends only on the barrier characteristics
-
(c)
Permeability is the speed with which molecules can pass through a barrier.
-
(d)
The more hydrophilic the molecule, the lower its permeability.
-
(e)
Polar molecules have higher permeability than nonpolar ones.
-
(a)
-
8.16.
Pick the correct statement.
-
(a)
Drugs transported by diffusion through membranes are restricted by the flow of solvent.
-
(b)
Drugs transported by diffusion through membranes are affected by the diffusion of other molecules.
-
(c)
Drugs can diffuse through the membrane in either direction.
-
(d)
Drugs diffuse through membranes up their concentration gradient.
-
(e)
Drugs transported by diffusion through membranes are trapped inside the cells.
-
(a)
-
8.17.
Which of the following statements about plasma membranes is wrong?
-
(a)
The exterior of the basolateral side of a plasma membrane is hydrophobic.
-
(b)
The exterior of the apical side of a plasma membrane is hydrophilic.
-
(c)
The exterior of a cell membrane is hydrophilic.
-
(d)
The interior of a cell membrane is hydrophobic.
-
(e)
The lipid component of cell membranes is mainly phospholipid.
-
(a)
-
8.18.
Choose the correct statement from below.
-
(a)
Diffusion is osmosis and advection.
-
(b)
Diffusion is advection and convection.
-
(c)
Advection is diffusion and convection.
-
(d)
Advection is solute convection.
-
(e)
Convection is advection and diffusion.
-
(a)
-
8.19.
Oxygen enters the lungs by __________ and the red blood cells by ____________.
-
(a)
Diffusion, advection
-
(b)
Diffusion, convection
-
(c)
Advection, diffusion
-
(d)
Advection, convection
-
(e)
Convection, advection
-
(a)
-
8.20.
Choose the correct statement referring to the concepts of diffusivity and permeability from the list below.
-
(a)
Diffusivity but not permeability is affected by temperature.
-
(b)
Permeability is the diffusion coefficient divided by the thickness of the membrane.
-
(c)
Permeability but not the diffusion coefficient is affected by the particle size of the solute.
-
(d)
The physicochemical properties of the solute affect the value of permeability but not the value of diffusivity.
-
(e)
The viscosity of a membrane affects the diffusivity of a solute but not its permeability.
-
(a)
-
8.21.
Select the statement that best defines the mass flux, J, of a substance.
-
(a)
J is the rate of substance mass that flows through a unit area.
-
(b)
J is the mass of substance that flows through a unit area.
-
(c)
J is the speed with which a substance flows through a membrane.
-
(d)
J is the easiness with which a substance goes through a membrane.
-
(e)
J is the mass of a substance that flows through a membrane per unit time.
-
(a)
-
8.22.
Given the following information, use the Fick’s equation to determine the rate of diffusion of the drug across a membrane.
Permeability coefficient of the drug = 0.006 cm/min
Surface area of the membrane between the two solutions = 3 cm2
Solution at apical side of the membrane = 0.15 M
Solution at basolateral side of the membrane = 0 M
Drug MW = 182 g/mol
-
(a)
0.0295 g/s
-
(b)
4.9·10−4 g/s
-
(c)
0.00049 mol/min
-
(d)
8.19·10−6 g/s
-
(e)
0.00049 g/min/cm2
-
(a)
-
8.23.
The graph below depicts the rate of diffusion of a drug across a biological membrane of surface area A = 2 cm2 and thickness equal to 10 nm. What is (a) the permeability in cm/min and (b) the diffusion coefficient of the drug in cm2/s?
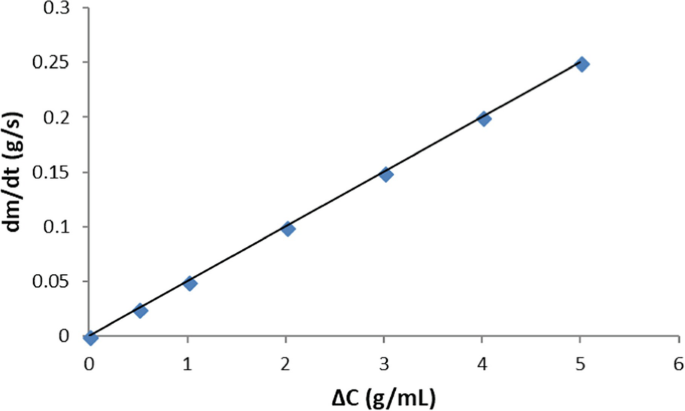
-
(Answer: (a) 1.5 cm/min; (b) \( 2.5\bullet {10}^{-8}\frac{{\mathrm{cm}}^2}{\mathrm{s}} \))
-
-
8.24.
Given the following information, use the Fick’s equation to determine the flux of the drug across a membrane in g/(min·cm2).
Permeability coefficient of the drug = 3.5·10−4 cm/s
Surface area of the membrane between the two solutions = 1.2 cm2
Solution at apical side of the membrane = 0.29 mM
Solution at basolateral side of the membrane = 0.02 mM
Drug solubility = 12 g/L
Drug MW = 254 g/mol
Log P = 2.1
pKa = 1.2
-
(a)
1.44 ∙ 10−6
-
(b)
0.024
-
(c)
9.45∙10−5
-
(d)
Cannot be calculated
-
(e)
0.00567
-
(a)
-
8.25.
Under constant experimental conditions, drug A has a permeability coefficient twice as big as that of drug B, but the MW of drug B is one half of the MW of drug A. Which drug has greater flux across the same membrane if both drugs are added only on one side of the membrane at the same concentration?
-
(a)
Both drugs have the same rate of diffusion.
-
(b)
Not enough information is given.
-
(c)
The rate of diffusion of drug A is smaller than that of drug B.
-
(d)
The rate of diffusion of drug A is bigger than that of drug B.
-
(e)
Impossible to compare because the rate of diffusion varies with time.
-
(a)
-
8.26.
Four in vitro cell monolayer permeation experiments are shown below at time zero. Answer the following assuming that the volumes of the apical and basolateral compartments are equal, and each solid hexagon represents one drug molecule.
-
(a)
Rank the experiments from higher to lower mass drug flux.
-
(b)
Rank the experiments from higher to lower apical-to-basolateral mass drug flux.
-
(c)
How would these experiments look like at equilibrium? Draw or describe your answer.
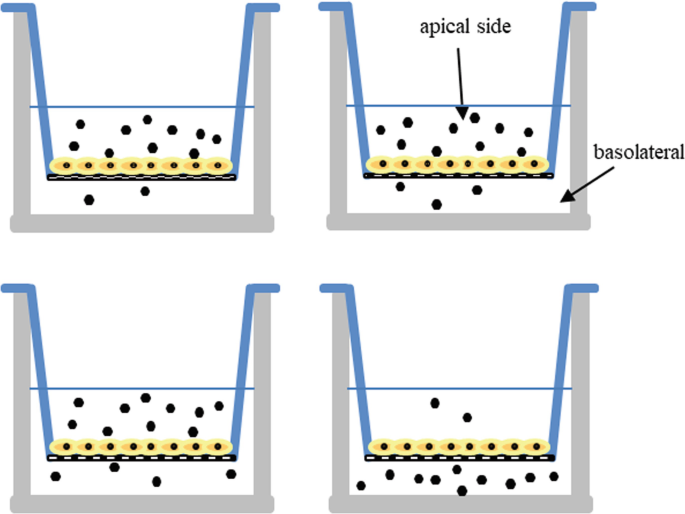
-
(a)
-
8.27.
Two permeation experiments were conducted to compare the apical-to-basolateral flux to the basolateral-to-apical drug flux. As shown below, the ratio of apical to basolateral drug concentration at time zero was 5/1 (left) versus 1/5 (right). At the end of the experiment, it was found that the basolateral-to-apical flux was 2.5 times bigger than the apical-to-basolateral flux.
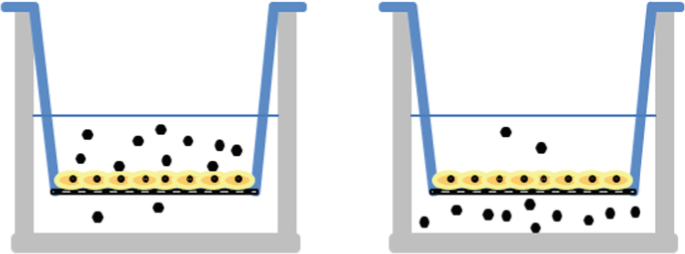
-
(a)
Are these the results that you would have expected?
-
(b)
Can you provide an explanation for these results?
-
(a)
-
8.28.
Four different drugs were classified as class I, II, III, and IV, respectively, according to the Biopharmaceutical Classification System and were formulated as immediate release tablets for oral administration.
Class
Solubility
Permeability
I
High
High
II
Low
High
III
High
Low
IV
Low
Low
Use the concept of permeability and concentration gradient to answer the following:
-
(a)
Which drugs have a potential to be absorbed from the GI via passive diffusion?
-
(b)
Which drugs would you recommend to administer with food?
-
(a)
-
8.29.
Which of the following statements is wrong regarding the passive diffusion of drugs through intestinal membranes?
-
(a)
In transcellular transport, drugs diffuse through the core of cells.
-
(b)
Transcellular diffusion is about the same in all tissues.
-
(c)
In paracellular transport, drugs pass through the water-filled gaps between adjacent cells by convection.
-
(d)
Paracellular drug transport varies in different tissues.
-
(e)
Paracellular transport is in general more efficient than the transcellular diffusion of drugs.
-
(a)
-
8.30.
Which of the molecular properties or characteristics below does not favor paracellular transport of drugs?
-
(a)
High surfactant activity
-
(b)
High hydrophilicity
-
(c)
High polarity
-
(d)
Low MW
-
(e)
Small molecular dimensions
-
(a)
-
8.31.
During an osmosis experiment, the amount of liquid increases on the side of the membrane with the greater ___________. Choose all that apply.
-
(a)
Solute concentration
-
(b)
Water concentration
-
(c)
Hydrostatic pressure
-
(d)
van’t Hoff ionization factor
-
(e)
Osmolarity
-
(a)
-
8.32.
Choose the correct statement. During an osmosis experiment, water diffuses from:
-
(a)
One isosmotic solution to another
-
(b)
An isosmotic solution to a hypoosmotic solution
-
(c)
A hyperosmotic to an isosmotic solution
-
(d)
A hyperosmotic to a hypoosmotic solution
-
(e)
A hypoosmotic to a hyperosmotic solution
-
(a)
-
8.33.
Choose the correct statement.
-
(a)
In osmosis, the solvent molecules diffuse from high drug concentration to low drug concentration.
-
(b)
In osmosis, the solvent molecules diffuse from low drug concentration to high drug concentration.
-
(c)
In osmosis, the drug molecules diffuse from high drug concentration to low drug concentration.
-
(d)
In osmosis, the drug molecules diffuse from low to high drug concentration.
-
(e)
In osmosis, both drug and solvent molecules diffuse from high drug concentration to low drug concentration.
-
(a)
-
8.34.
How is osmosis different from passive diffusion?
-
8.35.
Choose the correct statements about the absorption of a drug which is a substrate of both intestinal epithelium uptake and efflux protein transporters.
-
(a)
Uptake transporters located on the intestinal epithelium can only increase drug absorption.
-
(b)
Uptake transporters on the basolateral side of intestinal epithelium can only decrease drug absorption.
-
(c)
Uptake transporters on the apical side of the enterocytes can only increase drug absorption.
-
(d)
Efflux transporters of the intestinal epithelium can only decrease drug absorption.
-
(e)
Efflux transporters on the apical side of intestinal epithelium can only increase drug absorption.
-
(a)
-
8.36.
A drug is a substrate of both uptake and efflux transporters located on renal epithelium. Choose the correct statements that describe the role of protein transporters in urinary drug excretion. Remember that according to the general rule, the apical side of renal epithelial cells is facing the lumen or renal tubule (where urine forms), whereas the basolateral side of renal epithelial cells is facing the blood circulation.
-
(a)
Uptake transporters located on the renal epithelium can only increase drug excretion.
-
(b)
Uptake transporters on the basolateral side of renal epithelium can only decrease drug excretion.
-
(c)
Uptake transporters on the apical side of renal epithelium can only increase drug excretion.
-
(d)
Efflux transporters located on the basolateral side of renal epithelium can only decrease drug excretion.
-
(e)
Efflux transporters on the apical side coupled with uptake transporters on the basolateral side of the renal epithelium can maximize drug excretion.
-
(a)
-
8.37.
Given a 27.5% w/v aqueous sucrose solution of density 1.10 g/mL, determine the following:
-
(a)
Sucrose % w/w
-
(b)
Water % w/w
-
(c)
Water % w/v
-
(Answer: (a) 25% w/w; (b) 75% w/w; (c) 82.5% w/v)
-
-
(a)
-
8.38.
Given a 15% w/v aqueous glycerol solution of density 1.10 g/mL, determine the following if the MW of water and glycerol are 18 and 92 g/mol, respectively.
-
(a)
Glycerol molarity
-
(b)
Glycerol molality
-
(c)
Water % w/v
-
(d)
Water molarity
-
(Answer: (a) 1.63 M; (b) 1.716 m; (c) 95% w/v; (d) 52.78 M)
-
-
(a)
-
8.39.
Two aqueous solutions, dextrose 2% w/w (d = 1.08 g/mL) and glucose 7% w/v (d = 1.25 g/mL), are separated by a biological membrane at physiological temperature (37 °C). The MW of dextrose and H2O are 180 and 18 g/mol, respectively. Assuming that the membrane is only permeable to water molecules, calculate using molalities:
-
(a)
The osmotic pressure of the system.
-
(b)
If you are to mix these solutions to prepare 1 L of isotonic dextrose solution, how much volume of each should you use?
(Answer: (a) 5.56 atm; (b) 413 mL and 587 mL of 2% w/w and 7%w/v, respectively)
-
(a)
-
8.40.
A mammalian cell of volume 0.6 nL and an intracellular fluid composition of 6 mM [Na+], 154 mM [K+], and 4 mM [Cl−] is exposed to an extracellular NaCl solution composed of 132 mM [Na+], 102 mM [Cl−], and 5 mM [K+] at physiological temperature (37 °C). Assume that the extracellular volume is much bigger than the cell volume, Na+, K+, and Cl− are the only solutes inside and outside of the cell, the membrane is impermeable to the ions, and the cell has no ion channels or active pumps. Calculate:
-
(a)
The initial osmotic pressure in the system
-
(b)
The cell volume at equilibrium
-
(Answer: (a) 1.91 atm M; (b) 411.2 pL)
-
-
(a)
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Savva, M. (2019). Diffusion. In: Pharmaceutical Calculations. Springer, Cham. https://doi.org/10.1007/978-3-030-20335-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-20335-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20334-4
Online ISBN: 978-3-030-20335-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)




