Abstract
Twelve different units of concentration that are commonly used in pharmacy, chemistry, and biology, plus the salt factor, are defined both descriptively and mathematically in this chapter. Conversions of concentration units are demonstrated in great detail with practice examples. A vast number of practice exercises extracted from the area of protein research and other ingenious uses of drugs in hospitals to treat patients unresponsive to conventional treatments were created for you to become proficient in concentration unit conversions. Also, practice problems describing prescriptions that can be used to treat diseases such as psoriasis, arthritis, cholesterolemia, skin hyperpigmentation, and acne will keep you locked on a very interesting text that relates units of concentration with formulation creativity, pharmacological activity and toxicity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Author information
Authors and Affiliations
4.1 Electronic Supplementary Material
Units of concentration in pharmaceutical sciences. Description: (a) Differences between the concepts of density and concentration. (b) Define solute, solvent and solution. (c) Define various concentration units used in pharmaceutical sciences (MP4 889172 kb)
Applications of units of concentration. Description: (a) Definitions of concentration units is used to solve selected compounding exercises with emphasis on specific gravity of solute and specific gravity of solution, and conversion of w/v to w/w or vice versa (MP4 708333 kb)
Salt factor. Description: (a) Define the concept of salt factor. (b) Understand when to use and when not to use the salt factor. (c) Use the salt factor to normalize drug dosages (MP4 518517 kb)
Exercises
Exercises
-
4.1.
You have to compound the following prescription for a patient who suffers from seborrheic dermatitis and dandruff.
℞
Coal tar solution USP
5% w/v
Laureth-23
1 g
Laureth-4
2 mL
Alcohol USP
12% v/v
Isopropyl alcohol
4% v/v
Benzalkonium chloride
2.5% w/v
Purified water
q.s. ad.
946 mL
M. et ft. shampoo
(Coal tar topical solution USP, 5% is equivalent to 1% w/v coal tar; specific gravity: alcohol USP = 0.86; isopropyl alcohol = 0.785; Laureth-4 = 0.95; ethanol = 0.79)
-
(a)
How much coal tar is needed to fill the prescription?
-
(b)
How many milligrams of Laureth-4 are needed to make the shampoo?
-
(c)
How many milliliters (mL) of alcohol USP are needed to fill the prescription?
-
(d)
How many grams of isopropyl alcohol are needed to fill the prescription?
-
(e)
How much benzalkonium chloride is there in 4 fξ of shampoo?
-
(Answer: (a) 9.46 g; (b) 1900 mg; (c) 113.5 mL; (d) 29.7 g; (e) 2.957 g)
-
-
(a)
-
4.2.
Convert 4 ng/mL of drug Y into:
(a) ng/μL | (b) μg/μL | (c) μg/mL | (d) mg/μL |
(e) mg/mL | (f) mg/L | (g) g/μL | (h) g/dL |
(i) g/L | (j) gr/fξ | (k) oz/mL | (l) % w/v |
(m) Ratio strength | (n) ppm |
-
4.3.
You need to prepare 50 mL of 6 M solution of urea (H2NCONH2) for a protein denaturation experiment. How much urea do you have to weigh?
-
(Answer: 18 g)
-
-
4.4.
The levels of mercury (Hg ) in a patient’s urine was found to be 25 μg/dL. Assess whether the patient should be given dimercaptol if treatment with an antidote is recommended with mercury urine levels above 0.025 ppm.
-
(Answer: Mercury levels in urine = 0.25 ppm. The patient should be given the antidote)
-
-
4.5.
How many moles of phosphorus are there in 25 mL of 1.5 mM phosphoric acid? (AW P = 31)
-
(Answer: 3.75∙10−5 moles)
-
-
4.6.
Calculate milliliters of eucalyptus oil present in 2 oz of a 3% ointment if the specific gravity of eucalyptus oil is 0.93.
-
(Answer: 1.83 mL)
-
-
4.7.
How much ampicillin is needed to prepare 12 tablespoonful doses if the concentration of ampicillin in the syrup is 125 mg/5 mL?
-
(Answer: 4.5 g)
-
-
4.8.
Express the alcohol USP in units of molarity. (ρabs. ethanol = 0.79)
-
(Answer: 16.315 M)
-
-
4.9.
How much sodium chloride is required to make 1 L of 0.9% w/v solution for an i.v. infusion?
-
(Answer: 9 g)
-
-
4.10.
Calculate ng of cholesterol in the blood of a patient whose cholesterol blood levels are 240 mg/dL. Assume a 5 L blood volume.
-
(Answer: 1.2∙1010 ng)
-
-
4.11.
Calculate the equivalents present in 45 mL of 1.2 M H2SO4.
-
(Answer: 0.108 Eq)
-
-
4.12.
The concentration of eucalyptus oil in an emulsion is 3% w/w. Calculate the oil present (g and mL) in 3 fξ of emulsion if the density of eucalyptus oil is 0.93 g/cm3 and specific gravity of the emulsion is 1.12.
-
(Answer: 2.98 g; 3.2 mL)
-
-
4.13.
How many grams of alcohol are present in 77 mL of 20 M ethanol? (MWethanol = 46)
-
(Answer: 70.84 g)
-
-
4.14.
Calculate how much PLO (pluronic lecithin organogel) is needed to prepare 256 g of nifedipine gel 2% w/w.
-
(Answer: 250.88 g)
-
-
4.15.
Given the following formula:
Salicylic acid
6 g
Neutrogena clear pore®
6 fξ
Water
q.s. ad
8 fξ
Calculate the % w/v of salicylic acid in the formula if the Neutrogena clear pore®, the specific gravity of which is 1.43, contains 2% w/w salicylic acid.
-
(Answer: 4.68% w/v)
-
-
4.16.
Witch hazel commonly used as an astringent for the treatment of hemorrhoids is provided as a 14% v/v alcohol extract. Convert to % w/v if the specific gravity of the witch hazel is 0.98.
-
(Answer: 13.72% w/v)
-
-
4.17.
According to the standards of The National Cholesterol Education program, a HDL level below 35 mg/dL is considered low, indicating increased risk of heart disease. You ran an ELISA test on a 50 μL blood sample of a patient who has a heart disease and you detected 38 μg HDL present in the blood sample. Is his HDL low or high according to the standards?
-
(Answer: High, 76 mg/dL)
-
-
4.18.
How many grains of betamethasone are there in 8 oz. of 1.5% betamethasone ointment?
-
(Answer: 52.34 g)
-
-
4.19.
Calculate how many milligrams of Nafcillin Sodium are needed to prepare 40 μL of 125 mg/mL Nafcillin solution.
-
(Answer: 5 mg)
-
-
4.20.
How many grams of sodium chloride should a pharmacist use to make 1 L of 150 mM NaCl solution? (A.W.: Na = 23; Cl = 35.5)
-
(Answer: 8.775 g)
-
-
4.21.
How many grams of KCl are required to make 750 mL of solution whose molarity is equal to that of 5% dextrose. (MWs: dextrose = 180; KCl = 74.5)
-
(Answer: 15.52 g)
-
-
4.22.
In preparing the following solution:
Drug X | (10% w/v) | ||
Drug Y | (4% w/v) | aa | 25 mL |
Cherry syrup | q.s. ad | 90 mL |
-
(a)
How many grams of drug X and how many grams of drug Y are there in the formula?
-
(b)
Calculate the percentage strength of drug X in the final solution.
-
(c)
What is the ratio strength of drug Y in the final solution?
-
(Answer: (a) X = 2.5, Y = 1 g; (b) 2.78% w/v; (c) 1:90 w/v)
-
-
4.23.
You have to fill a prescription that calls for 2 oz of cream containing 0.01% w/w betamethasone for psoriasis treatment. You do not have the pure drug but you have a 1% betamethasone cream. How much of 1% betamethasone cream is required to fill the prescription? What is the percentage error associated with the weighing if the sensitivity of the balance is 10 mg?
-
(Answer: 0.567 g; 1.76%)
-
-
4.24.
Calculate the salt factor of hydroxyzine pamoate (Vistaril) and hydroxyzine HCl (Atarax). (MW (hydroxyzine) = 375, MW (hydroxyzine pamoate) = 745, MW (hydroxyzine HCl) = 411)
-
(Answer: 0.50, 0.91)
-
-
4.25.
Calculate the salt factor of metoprolol tartrate.
(MW (metoprolol) = 267.4; MW (metoprolol tartrate) = 684.8)


-
4.26.
A 45-year-old, 81 kg male patient with chronic bronchitis requires therapy with oral theophylline. The recommended empirical theophylline dosage is 0.4 mg/kg/h. Given the following structures and the periodic table, what is the equivalent aminophylline 8-h dose for the recommended theophylline dose?
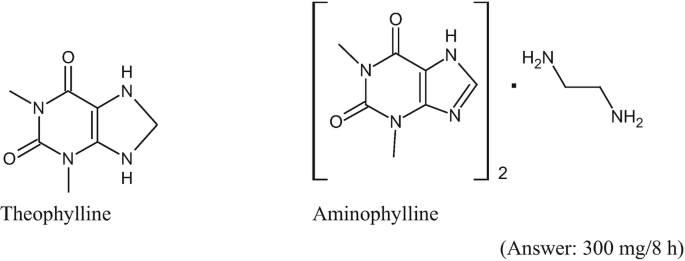
-
4.27.
How many grams of benzoyl peroxide paste are needed to prepare 60 g of 10% benzoyl peroxide ointment if benzoyl peroxide paste is supplied as 90% benzoyl peroxide and 10% dibutyl phthalate.
-
(Answer: 6.67 g)
-
-
4.28.
Transdermal nifedipine can be used to accelerate healing by inducing localized vasodilation without systemic effects. Given the prescription below:
℞ | ||
|---|---|---|
Nifedipine | 8% | |
Pluronic lecithin organogel | q.s ad. | |
Sig. Apply 80 mg nifedipine on the wound b.i.d. | ||
-
(a)
How many grams of the dosage form should you prepare for a 30 day supply?
-
(b)
Calculate how much nifedipine is actually in the skin if the release of the drug from the gel to the wound after the first dose was only 0.21%.
-
(c)
The levels of nifedipine in the blood after 12 h (first trandermal dose) were found to be 0.020 ppm. Calculate how much of the applied dose (%) is in the blood assuming that the blood volume of the patient is equal to 5 L. (dblood = 1.03 g/cm3)
-
(Answer: (a) 60 g; (b) 0.168 mg; (c) 0.103 mg, 0.129%)
-
-
4.29.
Lead (Pb) blood levels in the range of 80–100 μg/dL can cause encephalopathy in children. Express the concentration of lead in the blood as ppm.
-
(Answer: 0.8–1 ppm)
-
-
4.30.
Calculate the mole fraction of NaCl in 1 L of 0.9% solution. Specific gravity of the solution is 1.03. (AWs: Na = 23; Cl = 35.5; H = 1; O = 16)
-
(Answer: 0.0027)
-
-
4.31.
What is the percentage strength of betamethasone ointment if 4 g of betamethasone drug was added in 2 oz of 2.5% ointment?
-
(Answer: 8.925% w/w)
-
-
4.32.
The following prescription (milk of magnesia) was ordered for a 4½-year-old child who had no bowel movement for 4 days:
℞
Mg(OH)2
80 g/L
Water
q.s. ad
26 fξ
Directions for laxative use: Follow each dose with a full glass (236 mL) of liquid.
Milk of magnesia (Mg(OH)2 80 g/L) dosing chart:
Age
Dose
12 years and older
2–4 tbsp once a day
6–11 years
1–2 tbsp once a day
2–5 years
1–3 tsp once a day
Under 2 years
Ask a doctor
(AW: Mg = 24, O = 16, H = 1)
-
(a)
How much magnesium hydroxide do you need to weigh out in order to fill the prescription?
-
(b)
What is the normality of the solution?
-
(c)
How many moles of hydroxyl ions are there in a liter of solution?
-
(d)
Express the magnesium hydroxide concentration as ratio strength.
-
(e)
How many grams of magnesium ion are there per dose (give a range)?
-
(Answer: (a) 61.5 g; (b) 2.76 N; (c) 2.76 moles; (d) 1:12.5 w/v; (e) 0.1656–0.4968 g)
-
-
(a)
4.1.1 Additional Exercises
-
4.33.
A young adult is using salicylic acid 0.05%w/v solution for the treatment and management of acne.
-
(a)
Express the concentration of salicylic acid in 90 mL as ratio strength.
-
(b)
Calculate how much drug is needed to make 90 mL of solution.
-
(Answer: (a) 1: 2000 w/v; (b) 0.045 g)
-
(a)
-
4.34.
A 5%w/v coal tar topical solution USP is equivalent to 1% w/v coal tar. How much coal tar should you use to prepare 32 fξ of coal tar topical solution USP, 5%?
-
(Answer: 9.4624 g)
-
-
4.35.
If 7.5% w/w coal tar solution USP is equivalent to 1.75% w/w coal tar, how much coal tar is contained in 1 oz of coal tar solution?
-
(Answer: 0.496 g)
-
-
4.36.
The % w/v of a 5% w/w drug solution is 4. Calculate its specific gravity.
-
(Answer: 0.8)
-
-
4.37.
℞x
Xylometazoline HCl | 0.1% | |
Benzalkonium chloride | 0.09% | |
Isotonic saline | q.s ad. | 25 mL |
Sig. 2 drops in each nostril q.8 h. |
-
(a)
Calculate the ppm of benzalkonium chloride contained in the nasal decongestant?
-
(b)
How much xylometazoline is needed to fill the prescription?
-
(c)
Assuming that each drop is equivalent to 0.05 mL of solution, how much drug (μg) is in each dose per nostril?
-
(Answer: (a) 900 ppm; (b) 0.025 g; (c) 100 μg xylometazoline, 90 μg benzalkonium)
-
-
4.38.
Ampicillin sodium (2 g) was diluted with intravenous solution to 1 L for intravenous drip administration. After 2 h of storage at room temperature, the drug lost 10% of its activity.
-
(a)
Calculate the initial concentration of the drug in the IV bag.
-
(b)
How much drug has degraded in the IV bag after 2 h?
-
(c)
What is the concentration of the drug after 2 h?
-
(Answer: (a) 2 g/L; (b) 0.2 g; (c) 1.8 g/L)
-
-
(a)
-
4.39.
The following formula is used for treatment of acne:
Benzoyl peroxide | 10% w/v | |
Citric acid | 7 | |
Methyl paraben | 0.96 mg/mL | |
Propyl paraben | 0.02% w/v | |
Water | q.s. ad | 236 mL |
Mix and make lotion |
-
(a)
Calculate the amount (g) of benzoyl peroxide in the lotion.
-
(b)
What is the % w/v of methyl paraben in the lotion?
-
(c)
Express the concentration of propyl paraben in the lotion as mg/mL, ppm, and ratio strength.
-
(Answer: (a) 23.6 g; (b) 0.096%; (c) 0.2 mg/mL, 200 ppm, 1:5000 w/v)
-
-
4.40.
If you added 4 mL of 0.250 g/mL antibiotic solution to 1 L of 1.5% solution of the same antibiotic in 0.9% NaCl for parenteral infusion, what would be the % w/v of the antibiotic in the infusion solution?
-
(Answer: 1.59% w/v)
-
-
4.41.
A pediatric nasal spray contains 20 mL of xylometazoline HCl 0.05% and benzalkonium chloride 0.7%. The patient is to spray three times a day in each nostril.
-
(a)
Calculate the ppm of benzalkonium chloride (preservative) contained in the nasal decongestant?
-
(b)
Calculate the amount of drug and the amount of preservative per dose per nostril if the container is calibrated to deliver mist equivalent to 55 μL per spray.
-
(c)
How long is the medication going to last?
-
(Answer: (a) 7000 ppm; (b) 27.5 μg xylometazoline, 385 μg benzalkonium; (c) 60 days)
-
-
(a)
-
4.42.
The following formula can be used for wart removal:
Salicylic acid | 40% w/v | |
Mineral oil | 17 mL | |
Zinc oxide | ||
Talc | aa | 4 |
Ethanol 70% | q.s. ad | 1 fξ |
M. et ft. lotion |
-
(a)
Calculate ZnO in terms of % w/v.
-
(b)
How much salicylic acid is needed to make 2 fξ of the above formula?
-
(c)
How many grams of mineral oil are needed to make the lotion, given a specific gravity of mineral oil equal to 0.867.
-
(Answer: (a) 13.53%; (b) 23.656 g; (c) 14.74 g)
-
-
4.43.
The following formula can be used to make vitamin C effervescent tablets:
Ascorbic acid
10
Calcium carbonate
6.25
Sodium bicarbonate
13.1
Citric acid
10
M. et div. tabs no. x
Directions:
Adults: Place 1 tablet in 150 mL of water, let it dissolve (1 min) and drink
Children: ½ tablet
Chemical formulas:
Ascorbic acid C6H6O6; calcium carbonate CaCO3; sodium bicarbonate NaHCO3; citric acid (C6H8O7) or HOOC-CH-C(OH)(COOH)-CH-COOH
AW: C = 12, O = 16, Na = 23, Ca = 40, H = 1
-
(a)
How many millimoles of vitamin C are present per adult dose?
-
(b)
Calculate the normality of citric acid per adult dose assuming a full dissociation of citric acid in water.
-
(c)
How many moles of calcium ions (Ca+2) are there per child dose?
-
(d)
Calculate the mole fraction of sodium bicarbonate in the powder mixture.
-
(Answer: (a) 5.75 mmol; (b) 0.104 N; (c) 0.003125 mol; (d) 0.475)
-
-
(a)
-
4.44.
The same patient of Example 4.10 went back to her orthopedics physician and asked him if he could prescribe her a “beverage” instead of the powder form, so that she could have the magic potion while driving at work. The physician asked your help. You naturally came up with a solution within a minute:
℞
Glucosamine
0.25
Vitamin C
8
Chondroitin
3.2
α-Lipoic acid
16 mg
Sucrose
11.2
Water
q.s. ad
16 fξ
M. et ft. solution, div. # XVI
Sig. Drink q.d.
(MWs: vitamin C = 176 g/mol; sucrose = 342 g/mol)
-
(a)
What capacity dosage bottles should you use?
-
(b)
Calculate the % w/v of chondroitin in the solution.
-
(c)
Determine ppm of α-lipoic acid per dose.
-
(d)
Calculate the molarity (M) of vitamin C per dose.
-
(e)
Calculate the normality of sucrose in a dose.
-
(Answer: (a) 1 fξ; (b) 0.676%; (c) 33.8 ppm; (d) 0.096 M; (e) 0.069 N)
-
-
(a)
-
4.45.
A patient is to be infused with 1 L of solution containing 23 μg/mL of drug Z in 5% dextrose. (MW: drug Z = 560; glucose = 180; H2O = 18; specific gravity of the solution = 1.05)
-
(a)
Calculate the molarity of the drug Z.
-
(b)
What is the molarity of dextrose?
-
(c)
Calculate the molality of drug Z.
-
(d)
Calculate the mole fraction of dextrose.
-
(e)
Express the concentration of drug Z as ratio strength.
-
(Answer: (a) 4.11∙10−5 M; (b) 0.278 M; (c) 4.11∙10−5 m; (d) 0.005; (e) 1:43478 w/v)
-
-
(a)
-
4.46.
Concentrated sulfuric acid is supplied by the chemical companies as a 96% solution. (MW: H2SO4 = 98; H2O = 18; specific gravity = 1.86)
-
(a)
Calculate the molality of the solution.
-
(b)
What is the molarity of the solution?
-
(c)
Calculate the mole fraction of sulfuric acid.
-
(d)
Calculate the normality of the solution?
-
(Answer: (a) 244.9 m; (b) 18.2 M; (c) 0.815; (d) 36.4 N)
-
-
(a)
-
4.47.
Preparation of simulated gastric fluid requires addition of 10 mL of H2SO4 1 N to adjust the pH. You have on hand H2SO4 96%. How many milliliters of the concentrated sulfuric acid solution you have to use in order to adjust the pH? (MW: H2SO4 = 98; H2O = 18; specific gravity = 1.86)
-
(Answer: 0.275 mL)
-
-
4.48.
You wish to prepare the following eye wash formula:
H3BO3 | 2.1% w/v | |
Purified water | q.s. ad | 4 fξ |
-
(a)
How much boric acid is needed to compound the formula?
-
(b)
What is the molarity of the eye wash solution?
-
(c)
Calculate the normality of the solution assuming a full dissociation of the boric acid in water.
-
(Answer: (a) 2.48 g; (b) 0.340 M; (c) 1.020 N)
-
-
4.49-4.52.
Keratinocytes of psoriatic patients exhibit lack of differentiation and hyperproliferation consistent with significantly reduced levels of vitamin D3. The 1,25-dihydroxy-D3 and its analogs tacalcitol (1,24-dihydroxy-D3) and calcipotriene are used for the treatment of psoriasis.
-
4.49.
Given the prescription below:
℞ | |
|---|---|
Tacalcitol | 4 μg/g |
Glycerol | 0.4 g |
Pet. Album | 3 oz |
Sig. Apply on the affected area b.i.d. and h.s. | |
-
(a)
Calculate μL of glycerin required to fill the prescription. (ρglycerin = 1.25)
-
(b)
Calculate the mg of tacalcitol needed to fill the prescription.
-
(Answer: (a) 320 μL; (b) 0.342 mg)
-
-
4.50.
℞
Calcipotriene | 50 ng/μL | |
Glycerin | 10% v/v | |
ZnO | ||
Talc | aa | 4 g |
Isopropyl alcohol 70% | q.s. ad | 60 mL |
Sig: Apply 1 mL with a syringe on the affected area |
-
(a)
How much calcipotriene is required to fill the prescription?
-
(b)
How much (μg) calcipotriene is present in five doses?
-
(c)
How many grams of glycerin are needed to fill the prescription if the specific gravity of glycerin is 1.25?
-
(d)
Calculate the % w/v of ZnO in the lotion?
-
(Answer: (a) 3 mg; (b) 250 μg; (c) 7.5 g; (d) 6.67%)
-
-
4.51.
Given the following prescription:
℞ | ||
|---|---|---|
Calcipotriene | 50 ng/μL | |
Mineral oil | 15 mL | |
Propylene glycol | 4% v/v | |
Acacia | 15 g | |
Sodium phosphate dibasic | 0.852 | |
Water | q.s. ad | 60 mL |
Sig: apply 1 mL with a syringe on the affected area | ||
-
(a)
How much calcipotriene is required to fill the prescription?
-
(b)
How many grams of propylene glycol are needed to fill the prescription if the specific gravity of propylene glycol is 1.04?
-
(c)
Calculate the grams of mineral oil per dose given that the specific gravity of the oil is 0.925.
-
(Answer: (a) 3 mg; (b) 2.496 g; (c) 0.231 g)
-
-
4.52.
Given the following prescription:
℞ | |
|---|---|
Minocycline | 0.050 g/15 mL |
Cherry syrup | q.s. |
Sig. i tsp. b.i.d. and h.s. | |
-
(a)
How many milligrams of minocycline are in one dose?
-
(b)
Calculate the amount of minocycline required for 1 month (30 days).
-
(c)
How much cherry syrup is required to make 30 doses?
-
(Answer: (a) 16.67 mg; (b) 1.5 g; (c) 150 mL)
-
-
4.53.
℞
Menthol
0.15
Phenol liquified
0.5
ZnO
Talc
aa
4.0
Propylene glycol
11.0
2-Propanol
3 fξ
(Assume: 1 fξ = 30 mL)
Specific gravity of: 2-propanol = 0.79; phenol = 0.95; propylene glycol = 1.25
Ingredient quantities are in grams unless otherwise specified.
In preparing the anticeptic lotion above, you weighed ZnO with a 1.5% error. What is the concentration of ZnO (% w/w) in the lotion?
-
(Answer: 4.47% w/w)
-
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Savva, M. (2019). Units of Concentration and the Salt Factor. In: Pharmaceutical Calculations. Springer, Cham. https://doi.org/10.1007/978-3-030-20335-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-20335-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20334-4
Online ISBN: 978-3-030-20335-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)




