Abstract
Avian Influenza, commonly known as Bird Flu, is an epidemic caused by H5N1 Virus, that primarily affects birds such as chickens, wild water birds, ducks, and swans etc. On rare occasions, pigs and humans will also be affected with this virus In recent years this epidemic has emerged as a major global health concern. The present chapter is aimed at developing mathematical models that predict the spread and outbreak diversity of low pathogenic avian influenza virus. Essentially, we present (1) a deterministic mathematical model which deals with the dynamics of human infection by avian influenza both in birds and in human, (2) a discrete dynamical model for the spread of H5N1, and (3) the statistical transmission model of bird flu taking into account the factors that affect the epidemic transmission such as source of infection and social and natural factors.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
In the new millennium, the world has seen the emergence of three novel human respiratory viruses: SARS virus (a novel corona virus) in 2003, Avian Influenza virus (H5N1) in 2004 and an international outbreak caused by a new strain of influenza virus 2009 A (H1N1). This novel influenza 2009 A/H1N1 virus contains a combination of swine, avian, and human influenza virus genes. In sharp contrast to H5N1 and SARS viruses which emerged from the Asian continent, H1N1 virus emerged from North America (Mexico). Many important infectious diseases persist on a knife-edge: rapid rates of transmission coupled with brief infectious periods generate boom-and-bust epidemic that court extinction [5]. Such violent epidemic behavior has been observed in measles [14], cholera [33], meningitis [25, 58], and pertussis [49], among others. Several distinct mechanisms have been proposed to explain the spread and outbreak dynamics of avian influenza virus. These examples illustrate the need for understanding alternative spread and reinvasion mechanisms of infectious diseases for effective management and control.
In this chapter, we investigate the spread and outbreak dynamics of low pathogenic avian influenza virus. Avian Influenza, commonly known as Bird Flu, is an epidemic caused by H5N1 Virus, that primarily affects birds such as chickens, wild water birds, ducks, and swans etc. On rare occasions, pigs and humans will also be affected with this virus. Migratory aquatic birds, most notably wild ducks, are the natural reservoir of avian influenza viruses that inhabit the intestines of these birds. Infection in domestic poultry is thought to occur due to contact with these aquatic/wild birds. Fifteen subtypes of influenza virus are known to infect birds, providing a large pool of influenza viruses potentially circulating in bird populations. Avian Influenza is an infection caused by a virus known as orthomyxoviridae in virus classification. Influenza virus has only one species in it, which is called influenza A virus. Influenza A infects humans and animals such as birds, pigs, horses, and seals. When it infects birds, it is called avian influenza; when it infects pigs, it is called swine influenza, and so on. Typically, avian influenza viruses occur naturally among birds. But occasionally, avian influenza infects humans who have been in close contact with birds, with a rich pool of genetic and antigenic diversity that often leads to cross-species transmission. Wild birds worldwide carry these viruses in their intestine but usually do not get sick from them. However avian influenza is very contagious among birds and can make some domesticated birds including chickens, ducks, and turkeys very sick and kill them. Infected birds shed influenza viruses in their saliva, nasal secretions, etc. Susceptible birds become infected when they come in contact with the contaminated surfaces. Domesticated birds may become infected with avian influenza viruses through direct contact with infected waterfowl or other infected poultry or through contact with surfaces (such as dirt or cages) or materials (such as water or food) that have been contaminated with the virus [1, 14, 54].
Influenza A in humans is mainly a respiratory virus that typically infects cells of the nose and throat, but it can infect lung cells. It spreads when an uninfected person touches contaminated surfaces or inhales viruses coughed or sneezed out by an infected person. The recent avian strain that has been infecting humans is called H5N1 (named for its surface glycoproteins). H5N1 kills a high percentage of people who become infected, but thus far, the virus does not seem to spread well from person to person. Scientists fear that a person already infected with a human influenza A virus may become infected with the avian influenza A. If infected with both viruses, a hybrid avian–human influenza A virus may be generated that has two features making it particularly dangerous for human: avian H and N glycoproteins, to which humans have never been exposed (thus the immune system cannot quickly recognize and control the virus), and RNA from the human virus (enabling the hybrid to spread easily from person to person).
An outbreak of influenza A (H5N1) has been reported in several countries throughout Asia. Cases of Avian influenza A in birds have been confirmed in Cambodia, China, Hong Kong, Indonesia, Japan, Laos, Pakistan, Thailand, and Vietnam. Human cases of avian influenza have been reported in Thailand and Vietnam. During this outbreak investigation, it has been determined that avian influenza is spread from person to person. The current outbreak of avian influenza has prompted the killing of more than 25 million birds in Asia. In general the outbreak of an infectious disease is dependent upon three necessary conditions as the source of an infection, the route of transmission, and the herd susceptibility [32]. Other social and natural factors also play an important role in the transmission of infection, for example the control measures and the change in temperature. The source of infection that led to the outbreak is not clear. In some researcher’s view migratory birds are thought to be carrying the virus [9, 40]. If migratory birds had brought the virus one would have expected outbreaks well before February as bird migrations were over by around November. So we consider in our problem the source of outbreaks of bird flu to be the transportation of infected poultry as globalization has turned the chicken into the world’s number one migratory bird species. We have assumed that it is mainly due to the human activities of commerce and trade that spread this epidemic. We acquired some important information about bird flu such as the virus H5N1 is sensitive to temperature changes and the virus survives for shorter time at a high temperature. Also there are many effective control measures to block the virus transmission such as compulsory vaccination, culling of all infected or exposed birds [3]. Indeed, in the absence of such control mechanisms this avian influenza may pose a big threat to global health care [17, 29, 42, 45, 53, 61].
9.2 Some Biological Preliminaries
In this section, we present some material that helps one to understand the avian influenza H5N1 virus.
9.2.1 Viral Structure and Taxonomy
A virus, (an infectious agent that has DNA or RNA and protective covering) is a submicroscopic acellular particle that cannot survive in the absence of a living host cell. It relies on the host cell to replicate and cannot reproduce on its own. Since an antibiotic do not harm a virus, treatment for viral diseases such as flu mainly helps ease the symptoms rather than to kill the viruses. Most viruses cause generally mild diseases like the common cold and some even do not cause any symptom and may go unnoticed, but some cause diseases that can be severe and deadly like Avian influenza, AIDS, SARS, and some form of cancer [57].
The Avian influenza virus (AIV) contains negative (−) sense RNA as genetic material enters the host cell by attachment to the cell surface. Its hemagglutinin binds with the sialic acid present on glycoprotein receptors of the host. After adsorption, it is internalized as an endosome due to the acidic environment of the host cell. Influenza viruses are pleomorphic, mostly spherical or ovoid and filamentous, single-stranded RNA (ssRNA) enveloped viruses with a helical symmetry. They are covered over by lipid/lipoprotein envelope. The viral envelope has lipoprotein membranes that enclose nucleocapsids and nucleoproteins. It is endowed with an inherent capacity for genetic variation and is based on two important features: (1) the presence of a segmented genome, with 8 RNA segments that are genetically independent of each other and (2) a high rate of mutation, especially in the surface hemagglutinin (H) and neuraminidase (N) proteins. The diameter of each enveloped virus ranges from 50–120 nm and filamentous virions are 20 nm in diameter and 200–300 nm long. The genome is in the form of eight negative sense ssRNA fragments. The genome entire length is 12,000–15,000 nucleotides, the largest segment being 23–25 and the smallest being 800–900 nt. The longest RNA strand is closely associated with the nucleoprotein to form helical symmetry (see Fig. 9.1).
Schematic representation of influenza (flu) A virus [27]
There are some 500 distinct spike-like surface proteins of the viral envelope, each projecting 10–14 nm from the surface. There are mainly four types of glycoproteins/antigens:
-
1.
Hemagglutinin (HA) There are 16 types of HA reported.
-
2.
Neuraminidase (NA) There are nine types have been reported. The ratio of HA to NA is about 4–5 to 1.
-
3.
Nucleocapsid protein (NP) It coats the RNA strands.
-
4.
Matrix protein (M) The inner side of the viral envelope is lined by the matrix protein.
In Fig. 9.1, eight ribonucleoprotein segments (RNP) are surrounded by layer of matrix (M1) protein and lipid bilayer taken from host cell at budding. NS2 (NEP) protein is associated with M1. Three viral proteins are incorporated into the lipid bilayer: HA, NA, and M2 protein. HA trimers and NA tetramers form spikes on the surface of the virion. RNP segments contain viral RNA surrounded by nucleoprotein and associated with the polymerase complex [27].
Influenza A replication diagram in presented in Fig. 9.2 and we describe the replication process as follows:
Influenza A virus replication [4]
The virus loses its envelope in the cytoplasm, and the remaining core moves to the nucleus. Viral proteins are made in the cytoplasm using the host cell’s ribosome. In the cell cytoplasm the virus releases its nucleocapsids that further are transported into the nucleus, where mRNA synthesis and replication occurs. Once it enters the nucleus, viral endonuclease snips off. This snipped part of the host mRNA is used as a primer by the virus to synthesize its own mRNA. Next, viral RNA polymerase further extends the primer and makes a complementary (mirror image) plus (+) strand mRNA. Transcription results in eight primary transcripts/pre-mRNA that are further translated in the cytoplasm. The cells treat the viral mRNA like their normal mRNA and use them to make copies of viral proteins. The virus RNA is transcribed to messenger RNA in the nucleus. RNA replication occurs in the nucleus with the help of viral RNA polymerase that was also involved in transcription (see Fig. 9.2). In the same manner, the (+) strand of RNA (e.g., cRNA) is synthesized, and is coated with nucleocapsid proteins soon after it is made. This plus strand is then used as a template to synthesize a new negative RNA strand followed by coating with nucleocapsid proteins. These can further serve as templates for replication, mRNA synthesis, or packaging into virion particles. The (−) strand RNA (e.g., vRNA) are transported into the cytoplasm, where other viral proteins assemble together and are packed into virion particles and, on maturity, buds off from the outer cell membrane and infect new cells.
9.2.2 Classification of Influenza Virus
In general the influenza virus or flu virus can be classified into three categories: types A, B, and C which are distinguished by differences in two major internal proteins. Influenza virus type A is the most significant epidemiologically and the most interesting from an ecological and evolutionary stand point, because it is found in a wide variety of bird and mammal species [16, 55] and can undergo major shifts in immunological properties. Type B is largely confined to humans and very little is known about type C. Type A virus is responsible for causing Bird Flu, which was first found in Italy in 1878. Type A virus is further divided into subtypes based on differences in membrane proteins HA and NA, which are the most important targets for the immune system. The notation HhNn is used to refer to the subtype comprising the hth discovered HA proteins and the nth discovered NA protein. The subtype H5N1 virus of type A virus is the main cause of the bird flu [20, 26, 34, 43, 56]. Subtype is further divided into strains; each genetically distinct virus isolated is usually considered to be a separate strain [19, 47]. There are 16 types of HA surface proteins (which are named H1, H2, H3,…,H16) and nine types of NA surface proteins (which are named N1, N2,…,N9). An influenza virus always has one type of HA surface protein and one NA surface protein and it could be of any combination of H and N, e.g., H5N1, H7N3, H7N7, H5N2, H5N8, and so on [33, 35]. Within these subtypes, some viruses with slightly different nucleotide sequences are present and are classified into strains. The most virulent form so far reported is H5N1 of Influenza virus.
Influenza virus Type A can be divided into two distinct groups on the basis of their ability to cause disease. Highly pathogenic avian influenza (HPAI) can cause up to 100 % mortality in birds [1]. To date, all outbreaks of the highly pathogenic form have been caused by influenza A viruses of subtypes H5 and H7. We will mainly focus on Influenza virus Type A, the most virulent human pathogen and cause of all flu pandemics.
9.2.3 Epidemiology and Pathology
Infection with bird flu viruses in domestic poultry causes two main forms of disease that are distinguished by low and high extremes of virulence. The “low pathogenic” form may go undetected and usually causes only mild symptoms (such as ruffled feathers). However, the highly pathogenic form spreads more rapidly through flocks of poultry. This form may cause diseases that affect multiple internal organs and has a mortality rate that reaches 90–100 % often within 48 h [15].
The Avian Influenza virus (AIV) refers to Influenza A, found chiefly in birds. Infected birds show clinical symptoms like a sudden drop in egg production, brittle or soft-shelled and even shell-less eggs, congestion, swollen wattles and combs, and swollen skin under the eyes. The risk of human infection from birds is through coming in close contact with bodily fluids or with contaminated surfaces. Infection can be transmitted from infected bird droppings, saliva, nasal secretions, feces, or blood. These viruses can remain infectious for about one week at human body temperature or a month at 32 °F, and can survive at very low temperatures indefinitely. Symptoms of avian influenza in infected humans are mild fever, myalgia, myositis, and myoglobinuria. However, sore throat, cough, conjunctivitis; some people develop life-threatening complications like respiratory distress syndrome, pneumonia, and multiorgan failure.
In 1997, the first documented infection of humans with an avian influenza virus occurred in Hong Kong. At the same time, the poultry population in Hong Kong was also found to be infected with avian influenza caused by the same pathogenic strain. Studies determined that the infection occurred when the virus jumped directly from birds to humans due to close contact with infected poultry. A pandemic was averted by rapid mass killing/burning of over a million birds—the entire poultry population of Hong Kong [8, 46, 63]. For more than 2 years, the virus has ravaged poultry and caused human illness and death in many Southeast Asian countries and China. In December 2003, a highly pathogenic form of H5N1 caused another outbreak in poultry in South Korea [36]. Another human infection was confirmed in February 2004 when two fatal cases were reported in Hong Kong due to H5N1 [47], followed by 112 cases (57 fatal) from Thailand, Cambodia, Indonesia, and Vietnam. Between April and June 2005, a large number of wild water birds at Qinghai Lake in western China perished after being infected by the virus. During, July–August 2005, outbreaks involving virus were reported from Mongolia, Siberia, and Kazakhstan. The virus reached Turkey, Croatia, Romania, and Greece by October 2005. Ukraine reported outbreaks in November 2005. RnH5N1 viruses also have been isolated from ducks in Southern China [9, 10] and antiviral antibodies have been found in pigs in Vietnam [11]. The virus was infecting chicken and humans in northern Iraq by January 2006. In early February 2006 Nigeria became the first African nation to report the bird flu virus, with an outbreak at a large commercial poultry farm. In February 2006, many European countries, Egypt, and Iran found wild birds infected with H5N1 virus [20, 23, 26, 34, 37, 46]. These cases could be the result of new strains due to reassortant viruses, antigenic shift, or antigenic drift, as explained earlier. People are not immune to these different strains. Generally speaking, an individual has immunity to only those microbes or viruses to which they are earlier exposed. The possibility of dreadful new strains is thus worrying, as people either have no immunity or extremely delayed immunity depending upon the individual’s health and age. However, many new harmless strains causing symptomless infections go unnoticed. It was noticed that Spanish flu was most lethal in young adults, who generally are most able to fight off severe infections. One theory why Spanish flu preferentially killed young people is because they are the one with robust and reactive immune systems and therefore were most likely to mount a self-destructive response.
Avian influenza can result in immediate and severe disaster, for example the outbreak in USA in 1983–84 led to destruction of more than 17 million birds at a cost of nearly US$56 million [32]. Similar case again happened in Hong Kong in 1997–98 [22, 27, 53, 54]. Therefore rapid and effective measures must be taken to stop the spread of epidemics. The most effective measures to prevent the transmission of bird flu are rapid destruction of all infected or exposed birds, proper disposal of carcasses and excrement, quarantining and rigorous disinfectioning of farms and timely use of vaccine [7, 37, 52, 61].
Generally, the virus resides in bird droppings, contaminated soil, and airborne virus. Contaminated equipments, vehicles, food, cages, and clothing like shoes can carry the viruses from farm to farm. Some evidence suggests that flies can also act as mechanical vectors [30]. Wet markets where live birds are sold under crowded and sometimes unsanitary conditions can be another source of spread. These constitute the main cause of the former transmission. Export and import of poultry products are the main cause of the latter transmission, since they can carry the viruses for long distances freely when artificial factors are prevented. Migratory birds can also be a cause of transmission among the countries [9, 41]. Efforts have been made on the study of avian influenza and most of the recent papers focus on topics such as the route of transmission and physiological and biological properties. The bird flu virus of low pathogenicity can mutate into highly pathogenic one after a short time; the virus is sensitive to temperature change (it was found that the virus survives for shorter time at a higher temperature). This kind of influenza is able to transmit to humans under some circumstances; however no sufficient and clear evidences of human-to-human transmission are be found up to now [32].
9.2.4 Recent Efforts in the Control of H5N1
There are however some studies that aim at finding the possible drug-resistant H5N1 virus are reported in Wang et al. ([60] and the reference there in).
There has been a surge of interest in sensitive specific and rapid detection of avian Influenza virus in recent years and this helps one to find effective diagnosis and disease surveillance. Studies that aim at the detection of viruses by fluorescent DNA barcode-based immunoassay are available in the literature. It has been established that sensitivity of detection is comparable to conventional RT-PCR. For details we refer the readers to Cao et al. ([6] and the references there in).
A study on the first quantification of avian influenza virus in the organs of mute swans which died during the epizootic of H5N1 between January and April 2006 in the Czech Republic has been reported in Rosenbergova et al. [51]. For rapid detection and quantification of avian influenza virus RNA in clinical samples collected from mute swans are utilized to develop the quantitative real time Reverse Transcriptase PCR (qRT-PCR) assay based on a Taq Man Probe.
Though vaccines and antiviral are available that can provide protection from influenza infection, new viral strains emerge continuously due to the plasticity of the influenza genome. An alternative protection methodology that is based on the isolation of a panel of monoclonal antibodies derived from Ig G+ memory B cells of healthy human subjects that have a capability of recognizing new viral strains is discussed in Grandea III et al. [24].
From bioinformatics point of view to help the researchers develop methods to fight against H5N1 avian flu the following Website http://www.avian-flu.info is in vogue since 2004. For more details on this we refer the readers to Liu et al. [39].
9.3 Modeling Bird Flu
During the last decade, various mathematical models have been used for infectious diseases in general and for influenza in particular. In case of avian influenza, deterministic models were used for comparing interventions aimed at preventing and controlling influenza pandemics [13, 21], and stochastic model were proposed to model and predict the worldwide spread of pandemic influenza [12, 13]. In this section, we present (1) a deterministic mathematical model which deals with the dynamics of human infection by avian influenza both in birds and in human [18], (2) a one parameter model for spread of H5N1 [28] and (3) the statistical transmission model of bird flu taking into account the factors that affect the epidemic transmission such as source of infection, social and natural factors [59].
9.3.1 A Deterministic Mathematical Model
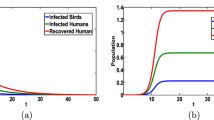
In human, consider SIRS compartmental model that is to say that human susceptible individuals become infectious then removed with temporary immunity after recovery from infection and susceptible when again immunity fades away, in bird population we consider SI compartmental model [18].
Let H and B denote the human and bird population sizes respectively. In this model death is proportional to the population size with rate constant μ and we assume a constant Ω due to births and immigrations. Therefore, \( \frac{ dH}{ dt}=\varOmega -\mu H \), whereas for bird population we suppose that B is constant. The human population (respectively bird population) of size H (resp. B) is formed of susceptibles S, of infective I and of removed R (resp. S 0 and I 0). βSI 0 /B is the human incidence that is, the rate at which susceptible become infective. If the time unit is days, then the incidence is the number of new infection per day. The daily contact rate β is the average number of adequate contacts of a human susceptible with infected birds per day and I 0 /H is the infectious fraction of the population. Time units of weeks, months, or years could also be used. Similarly β 0 S 0 I 0 /B is the bird incidence and β 0 is the average number of adequate contacts of a bird susceptible with other birds per day. The man life span is taken equal to 25,000 days (68.5 years), and the one for the bird is about 2,500 days (6.8 years).
The model is governed by the following equations:
Human population
Bird population
The continuity of the right hand side of this system and its derivatives implies that unique solutions exist on maximal interval. Since the solutions approach, enter or stay in a region of attraction R given by {(S, I, R, S 0, I 0, N) ∣S ≥ 0, S 0 ≥ 0, I 0 ≥ 0,S + I + R = H ≤ Ω/μ; S 0 + I 0 = B}, they are eventually bounded and hence exist for t ≥ 0. Therefore, the model is mathematically and epidemiologically well posed.
Introducing the nondimensionalized variables as
and with the conditions S + I + R = H and S 0 + I 0 = B,
The model systems (9.1) and (9.2) become:
in the set Ω′ = {(s, i, r, i 0)|s ≥ 0, i ≥ 0, r ≥ 0, s + i + r ≤ n ≤ 1; 0 ≤ i 0 ≤ 1}.
\( \mathrm{Let}\kern0.24em \tilde{R}=\frac{\beta_0}{\mu_0}. \) It has been shown in Derouich and Boutayeb [18] that the model (9.3) admits the trivial equilibrium (1, 0, 0, 0) if \( \tilde{R}\le 1 \) and this equilibrium is globally asymptotically stable, that is \( \underset{t\to \infty }{ \lim}\;i(t)=0 \) if \( \tilde{R}\le 1 \). Further the system (9.3) has an endemic equilibrium (\( \overline{s},\overline{i},\overline{r},{\overline{i}}_0\Big) \) in Ω′ if \( \tilde{R}>1 \) where
From the above results it follows clearly that the dynamics of the disease is mainly determined by the average number of adequate contacts of human susceptible with infected birds. For more details we refer the readers to [18].
9.3.2 A Discrete Dynamical Model [28]
In Eifert et al. [28], a one component discrete dynamical model for the spread of avian influenza is derived. This model utilizes Lindblad dissipation dynamics [2, 22, 38], for biological rate equation.
It is known in epidemiology that the hygienic stress of the virus ensemble plays an important role in the prevention of this virus. The viruses are damped in their replication rate by hygienic stress and prevention methods, provided these methods are intense. On the other hand if the hygienic stress is minimal, it will stimulate the virus replication.
The plain infection model without hygienic stress is given by
where x n denotes the relative number of infected being at time step n and “a” is the infection rate. Utilizing the methods suggested in [2, 22, 38], the authors in [28] have modified the model (9.4) incorporating the hygienic stress and assuming a power law relation [44]. The modified model becomes a special case of the following model
in which y is a fixed positive number, α > 0, β > 0 are real positive parameters. The coefficient in front with a power of a, indicates that the hygienic stress of the virus increases with the infection rate, and this power is given by βy. Clearly when the stress coefficient vanishes, model (9.5) should reduce to the plain infection model (9.4). In order that the model (9.5) to reduce to (9.4) in the limiting case, that is, when the stress coefficient vanishes, the term y α is introduced. The power law coefficient in front of the term x n ln (a x n ) describes the equation of state for the transport coefficient.
In [28], the authors have considered the model (9.5) with α = 1.5 and β = 50 (these numbers are chosen so that the probabilities x n stay positive and less than or equal to 1) and performed some numerical calculations. Since the true equation of state for the H5N1 virus is unknown, it is assumed that the hygienic stress coefficient is a monotone function of the infection rate a. Further model (9.5) reduces to (9.4) when y vanishes. Computer experiments have revealed that the infection probability is reduced by the hygienic stress and the onset of chaos is earlier as without any hygienic stress. The bifurcation diagrams that explain the spread of avian influenza H5N1 in respect of three specifically chosen stress functions, (y = 0.0, y = 0.07, and y = 0.1, 1.0) are presented in Figs. 9.3, 9.4, 9.5, and 9.6 (Bifurcation diagrams for the spread of avian influenza). For a detailed discussion on these numerics we refer the readers to Eifert et al. [28].
H5N1 without hygienic stress (May-Feigenbaum scenario for y = 0.0) [28]
H5N1 with weak hygienic stress (May-Feigenbaum scenario for y = 0.07) [28]
H5N1 with medium hygienic stress (May-Feigenbaum scenario for y = 0.1) [28]
H5N1 with strong hygienic stress (May-Feigenbaum scenario for y = 1.0) [28]
9.3.3 The Statistical Transmission Model
The mathematical model developed for the recent bird flu predicts that majority of the infection was in wild birds, market birds (includes backyard poultry birds taken to markets for selling) and farm birds, which play an important role in spreading the virus from the epicenter to the nearby centers in the region.
In order to study the patterns of the spread of epidemic, we have made an investigation of outbreaks of the epidemic in 1 week during February 13–18, 2006 till it reached India. On February18, 2006 the lethal strain of H5N1 virus surfaced in India in the trivial pocket of Navapur in Nandurbar District of Northern Maharashtra. It was the sixth day of our study. On Monday, the first day, outbreaks occurred in five countries of Africa and Eastern Europe namely Nigeria, Greece, Slovenia, Romania, and Bulgaria. On second day bird flu hit four nations of Southeast Asia, central Asia, and Europe namely Indonesia, Iran, Austria, and Germany. On third day, outbreaks took place in two European countries Hungary and Italy. On Thursday, new cases of bird flu were found in three European and African countries Switzerland, Denmark, and Egypt. Reaching France on Friday bird flu at last, on Saturday surfaced in India.
We know the major factors that play an important role in the transmission of bird flu are the way the infected poultry products are transported, air temperature, the control measures (for example, culling the poultry in the infected form, introducing compulsory vaccination to enhance the resistibility of poultry in the noninfected farms forbidding live birds being sold under crowded and unsanitary conditions), migratory birds, and other infected transportation vehicles (which means the vehicles carry infected poultry or bird dropping or contaminated soil, etc.). Also there are some other factors not considered in our model viz. bird flu transmitting to human beings and virus of low pathogenicity mutating into high pathogenicity after some time, since these elements will not contribute much to the usual transmission of bird flu.
How do these factors affect the transmission? The infected animals are the source of the infection, higher air temperature can drastically cut down the lifetime of the virus, and transportation of infected poultry is the route of transmission. Control measures such as active and effective actions play important role in preventing and destroying the epidemics, which can effectively block the route of transmission of the infection, diminish the source of infection and promote the resistibility of susceptible poultry. So we must take all the major factors into account in the formulation of our transmission model.
These factors may be reflected in the following parameters:
-
N (n) is the total number of regions of outbreak on the nth day.
-
D(n) is the lifetime of the virus regarding the nth day since the beginning of the epidemic which implicitly corresponds to air temperature.
-
I(n) is the resistibility of the poultry on the nth day since the beginning of the epidemic many of the above control measures objectively promote the resistibility of poultry and even human beings.
-
f(r) is the distribution of the probability that infected poultry products are transported a distance r.
The following are necessary assumptions for our model [59]:
Let P(n, r) represent the probability for a new outbreak to take place, then
Considering above assumptions, we obtain our proposed model for transmission of bird flu as under:
where
The parameter β is taken close to 0.6 and R ∈ [0,1] is a random float number.
9.3.3.1 Methodology
In this section, we discuss the methodology that was used for simulation experiments to study the model (9.6). We first discuss, one by one the various parameters taken in our model and explain how these parameters are and finally present the methodology used to predict a new possible outbreak.
-
1.
In our model we have used the random float number R which we have generated in our program by using random number generator. Why do we need R in our model? As we know the outbreak of bird flu is a probabilistic instance and not a deterministic one, even though f(r), D(n), N(n) contribute much to P(n,r) we cannot definitely assure that there will be a certain outbreak of bird flu in a region but can only say that there is enormous possibility or danger for an outbreak to take place. So an additional random parameter R is introduced to reflect the uncertainty.
-
2.
D (n) denotes the lifetime of the virus of bird flu on the nth day. The H5N1 virus can survive, at cool temperatures in contaminated manure for at least 3 months; in water the virus can survive for up to 30 days at 0 °C; about four days at 22 °C; about 3 h at 56 °C and only 30 min at 60 °C.
By means of fitting the above data by curve fitting method, we obtained an approximate formula as follows:
$$ D(t)={e}^{3.4-0.0915\kern0.24em {t}^{1.1}}, $$(9.7)where t represents the air temperature.
In our simulation, we study the epidemic of one week till it reached India that is for a short duration. Hence the temperature change may be taken as a linear approximation regarding the epidemic duration that is
$$ t(n)={t}_1+{t}_2n, $$(9.8)in which t 1 and t 2 are two constants that can be determined by fitting the average temperature of the various countries through which the virus reached India. In this model t 1 = 0.2 and t 2 = 2.2286 (started on February13, 2006). So the relation between the lifetime of the virus and the epidemic duration shall be a compound form of (9.7) and (9.8) as
$$ D(n)={e^{3.4-0.0915\left(0.2+2.2286n\right)}}^{1.1}. $$(9.9) -
3.
I(n) stands for the resistibility of poultry on the nth day. Obviously the resistibility will increase with the artificial interventions and the control measures. We assume the increase abides by law similar to sigmoid function 1/(1 + e − x). Thus I(n) assumes the following form
$$ I(n)=\frac{B}{1+\left(B-1\right){e}^{-n/C}}. $$(9.10)Apparently, this is a modified sigmoid form. When n = 0, I(0) = 1, and when n = ∞, I(n) = B; which indicates the resistibility is impossible to approach a very big number.
-
4.
f(r) is the distribution of the probability that infected poultry products are transported a distance r.
$$ f(r)={r}^{-\left(1+\beta \right)}. $$The basic idea for taking the above-mentioned form of f(r) stems from Howlett [31]. The researchers at Max Planck Institute for Dynamics and Self Organization, have used the dispersal of dollar bills within the United States as a proxy measurement of human movement (since people cannot be tracked while banknotes travel with the people). They analyzed the data on the peregrinations of more than half-a-million US dollar bills recorded over a 5-year period on an online bill-backing system and given a simple model that only depends upon two parameters. Since the poultry products are also imported and exported very frequently, to various countries, we can assume its transport to be very similar to the human movement. So the probability that infected poultry product is transported from the form may assume a distance r, for r larger than 10 km with β close to 0.6. This distribution behaves like a power law. This function decreases as r grows larger, meaning that transportation of poultry over a long distance is less common than short ones. However it does not decrease, as fast as other common probability distributions, which means that transportation over long distances, are still common enough to have a significant effect. Poultry products make many short journeys, but the occasional long haul ensures that they disperse widely.
-
5.
A threshold value S is necessary which acts as a criterion: when P(n,r) is greater than S there will be an outbreak; otherwise not. However, how to determine the possible number of outbreaks per day denoted by K(n)? Intuitively K(n) shall be in direct proportion to N(n), however since only the nearest several outbreaks have notable contribution to the probability of a new outbreak a number of distant outbreaks contribute little, so dependent relation of K(n) upon N(n) is of the form
$$ K(n)= AN{(n)}^b, $$(9.11)in which A ≥ 1 and 0 < b < < 1, that is, K(n) increases slowly with the augment of N(n).
Thus the method for predicting an outbreak is explained below.
Suppose the epidemic has begun, we compute the number of actual outbreaks on nth day. First we generate K(n) according to (9.11). Then we calculate each P(n,r) according to (9.6); when P ≥ S, a new outbreak will take place, otherwise not. So, the total number of new outbreaks is always less than K(n).
In our model, there are six parameters β, A, b, B, C, S which are all adjustable. So one may argue that too many adjustable parameters may not be an advantage for a “good” model. However, we must analyze independence of these parameters. Here S is not an independent parameter, rather dependent on f(r), hence on β. Parameter A controls the initial possible number of outbreaks the epidemic may abort if A is too small and overflow if A is large. So there shall be a proper intermediate value for A. The parameter b denotes the general trend of the outbreaks, the total number of outbreaks will grow too rapidly to be practical if b is a big number and may be too flat if b is too small. B determines the ultimate resistibility, which reflects the final degree of stringency of artificial interventions; the greater B is the more stringent the interventions are. As of C, it reflects the average degree of stringency throughout the epidemics; the smaller C is the more stringent the control measures are. In other words, B determines the final height of the curve I(n) and C controls the shape or the process of I(n). Therefore each parameter has a definite meaning and a specific role and has little overlap regarding the role, so the model is reasonable. For conducting simulations we have assumed distances <500 miles as 10 units, <500–1000 miles as 11 units, <1000–1500 miles as 12 units, and <1500–2000 miles as 13 units and so on.
9.3.3.2 Numerical Simulation Results
The parameters are initialized as at the beginning: N 0 = 6 refers to the total number of days (for which study is conducted) the epidemic lasts. N(1) = 5 means there are in all five cases of new outbreaks on the first day. The other parameters A, b, B, C, S will be determined through simulations by comparing with the actual epidemic data.
Results of simulation are shown in Figs. 9.7 and 9.8. The values of the related parameters are:
It is easy to see that the simulation is roughly in accordance with the actual situation. The parameters related to the artificial interventions include B and C. If B is big enough, few times can the epidemic take place in most simulations; if B is very small, the epidemic can always happen and most times the overflow can happen. As for C, if it is small enough, the epidemic hardly occurs, otherwise it can almost happen. Therefore the effective, powerful, and stringent control measures are the key to stop the epidemics.
It should be pointed out that the parameters in the model are independent of each other, since each of them plays an independent role—these parameters have definite meaning, so the result of simulation can hardly coincide with the actual situation of the epidemic if the parameters cannot be adjusted to proper values.
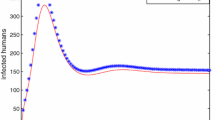
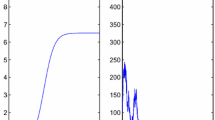
Simulation results are presented in Table 9.1. It is clear from the table that the probability of new outbreak to occur decreases with the increase of distance from the source countries. The probability of occurrence of outbreak in India is very low because the flu virus reaches on the sixth day and also the country is far from the source country. The probability of occurrence of outbreak in Indonesia is also very low even on the second day. This is due to very large distance from the source country and also due to some other factors (like temperature). Figures 9.7 and 9.8 show the pattern that the probability of outbreak to take place follows, depending upon the distance and number of days. These graphs show that the probability of outbreak is inversely proportional to the distance from the source country as well as the day on which the flu virus reaches the country.
Graph showing probability of outbreak is inversely proportional to the distance from the source country [59]
Graph showing the probability of outbreak is inversely proportional to number of days at a fixed distance [59]
9.4 Other Control Measures
The first and major control measure should be to take precautions so that an Avian Influenza virus does not cross the “species barrier” and infect human. If it crosses the “species barrier” and infects people, then the medical doctor should be contacted to take the proper medicine and suggestion for precautions. The persons dealing with poultry or poultry products should take the following prevention measures:
-
1.
Practice proper sanitation and good hygiene handling of poultry and poultry products,
-
2.
Wear masks, rubber gloves, and safety glasses,
-
3.
Avoid unnecessary contact with live, sick, or dead birds,
-
4.
Keep hands away from face and mouth when handling birds,
-
5.
Practice proper hand washing, cleaning and disinfection procedures, and avoid touching mouth or eyes. Indirect contact with door knobs, toilet knobs, taps, hand shaking etc., is another possible method of transmission,
-
6.
Wash eggs thoroughly with soap water, rinse, and thoroughly cook at the temperature more than 74 °C, cook chicken until boiling temperature or when cooking temperature exceeds 165 °F (74 °C), because H5N1 viruses are killed at this temperature,
-
7.
Store and prepare raw chicken and eggs separately from other food items to avoid cross contamination,
-
8.
Clean kitchen utensils and surfaces before and after use,
-
9.
There should be routine tests for AIV in poultry and if any positive case is seen, the entire poultry flock and poultry products should be destroyed and should not be consumed by the public at any cost.
-
10.
Report any unusual death or illness of chickens and other birds to the Dead Bird Hotline (888) 551-4636 and report illness among workers in poultry farms to the Health Department.
9.5 Discussion and Conclusions
Bird flu is a highly pathogenic epidemic that can result in serious disaster in many areas. Immediate and effective control measures are of great importance in preventing the transmission of avian influenza. So it is challenging to study the problem from various angles, and develop deterministic, discrete and statistical-mathematical transmission models. Using mathematical modeling, Breban et al. [5] have investigated the role of environmental transmission for the pattern and persistence of avian influenza in wild waterfowl and demonstrated that indeed environmental transmission is a fundamental ingredient for the modeling of this epidemic. The persistence mechanism induced by environmental transmission raises novel problems of epidemic control since traditional strategies may prove ineffective in the presence of an environmental viral reservoir [50]. For a global outbreak of influenza to occur, three conditions must be met: a new virus subtype must arise, this subtype must be able to cause serious illness in the human, and it must spread easily from person to person- and continue to do so. The first two conditions have been met with H5N1 human infections. Scientists are working for better understanding of different influenza A strains with the hope of preventing the spread of this virus. One of the reasons for the difficulty in predicting outbreaks is that migratory wild birds, not virus infected human carry the H5N1 virus long distance [62] (one cannot totally rule out the chance of human carrying bird flu for long distances).
Our ongoing investigations relate to the question as how to assess the actual control measures and assign the parameters in the model with proper numerical values. There is a need to strengthen the design of data collection and for advanced experimental facilities to understand the virus spread with respect to all seasons and food consumption patterns. Given the present situation, it is not easy for any government to predict accurately the timing and location of future avian influenza attacks; there is a vide scope to strengthen the databanks and to train relief teams to minimize economic loss, in the event that an outbreak occurs [48]. To complement these issues with public health side of research activities, the government could consider launching large-scale experimental projects to estimate various rates of spread of H5N1 in different geographical regions, and to come up with a comprehensive approach for effective control of an outbreak. Further modeling work is needed in order to explore the epidemiological dynamics and persistence of avian influenza viruses, with a view to understanding the respective roles of environmental transmission and demographic stochasticity.
References
Alexander DJ (2000) A review of avian influenza in different bird species. Vet Microbiol 74:3–13
Alicki R, Messer J (1983) Nonlinear quantum dynamical semigroups for many-body open systems. J Stat Phys 32:299–312
Anderson RM, May RM (1991) Infectious diseases of humans: dynamics and control. Oxford University Press, Oxford UK
Arias CF, Escalera-Zamudio M, Soto-Del Rıo Mde L, Cobia´n-Gu¨emes AG, Isa P, Lo´pez S (2009) Molecular anatomy of 2009 influenza virus A (H1N1). Arch Med Res 40:643–654
Breban R, Drake JM, Stallknecht DE, Rohani P (2009) The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comput Biol 5(4):1–11
Cao C, Dhumpa R, Bang DD, Ghavifekr Z, HØgberg J, Wolff A (2010) Detection of avian influenza virus by fluorescent DNA barcode-based immunoassay with sensitivity comparable to PCR. Analyst 135:337–342
Centers for Disease Control and Prevention (2004) Cases of influenza A (H5N1)-Thailand, 2004. MMWR Morb Mortal Wkly Rep 53:100–103
Chan PKS (2002) Outbreak of avian influenza A (H5N1) virus infection in Hong Kong 1997. Clin Infect Dis 34:s58–s64
Chen H, Smith GJD, Zhang SY, Qin K, Wang J, Li KS (2005) Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 436:191–192
Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P (2004) The evolution of H5N1 influenza viruses in ducks in southern China. Proc Natl Acad Sci U S A 101:10452–10457
Choi YK, Nguyen TD, Ozaki H, Webby RJ, Puthavathana P, Buranathal C (2005) Studies of H5N1 influenza virus infection of pigs by using virus isolated in Vietnam and Thailand in 2004. J Virol 79:10821–10825
Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A (2007) Modeling the world wide spread of pandemic influenza: baseline case and containment interactions. PloS Med 4:e13
Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A (2006) The modeling of global epidemics: stochastic dynamics and predictability. Bull Math Biol 68:1893–1921
Conlan AJK, Grenfell BT (2007) Seasonality and the persistence and invasion of measles. Proc Biol Sci 274:1133–1141
Cox NJ, Subbarao K (2000) Global epidemiology of influenza: past and present. Annu Rev Med 51:407–421
Cyranoski D (2005) Bird flu spreads among Java’s pigs. Nature 435:390–391
De Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL (1997) A pandemic warning? Nature 389:554
Derouich M, Boutayeb A (2008) An avian influenza mathematical model. Appl Math Sci 2:1749–1760
Earn DJD, Rohani P, Bolker BM, Grenfell BT (2000) A simple model for complex dynamical transitions in epidemics. Science 287:667–670
Ellis TM, Bousfield BR, Bissett LA, Dyrting KC, Luk GS, Tsim ST (2004) Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol 33:492–505
Ferguson NM, Fraser C, Donnelly CA, Ghani AC, Anderson RM (2004) Public health risk from the avian H5N1 influenza epidemic. Science 42(4):968–969
Gorini V, Frigerio A, Verri M, Kossakowski A, Sudarshan ECG (1978) Properties of quantum Markovian master equations. Rep Math Phys 13:149–173
Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M (2005) Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol 79:2191–2198
Grandea AG III, Olsen OA, Cox TC, Renshaw M, Hammond PW, Chan-Hui PY, Mitcham JL, Cieplak W, Stewart SM, Grantham ML, Pekosz A, Kiso M, Shinya K, Hatta M, Kawaoka Y, Moyle M (2010) Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci U S A 107(28):12658–12663
Greenwood B (2006) Pneumococcal meningitis epidemics in Africa. Clin Infect Dis 43:701–703
Guan Y, Peiris JSM, Lipatov AS, Ellis TM, Dyrting KC, Krauss S (2002) Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc Natl Acad Sci U S A 99:8950–8955
Gubareva LV, Kaiser L, Hayden FG (2000) Influenza virus neuraminidase inhibitors. Lancet 355(9206):827–835
Eifert H-J, Held S, Messer JA (2009) A one-parameter model for the spread of Avian Influenza A/H5N1, Chaos Soliton Fract 41(5):2271–2276
Hien TT, De Jong M, Farrar J (2004) Avian influenza—a challenge to global health care structures. N Engl J Med 351:2363–2365
Hope-Simpson RE (1992) The transmission of epidemic influenza. Plenum, New York
Howlett R (2006) Travel: fitting the bill. Nature 439:402
Li J, Ren Q, Chen Xi, Yin J (2004) Study on transmission model of Avian influenza. Proceedings of international conference on Information Acquisition, IEEE, p 54–58
King AA, Ionides EL, Pascual M, Bouma MJ (2008) Inapparent infections and cholera dynamics. Nature 454:877–880
Kuiken T, Rimmelzwaan G, Van RD, Van AG, Baars M, Fouchier R (2004) Avian H5N1 influenza in cats. Science 306:241
Lee CW, Senne DA, Suarez DL (2004) Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J Virol 78:8372–8381
Lee CW, Suarez DL, Tumpey TM, Sung HW, Kwon YK, Lee YJ (2005) Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol 79:3692–3702
Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L (2004) Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209–213
Lindblad G (1976) On the generators of quantum dynamical semi groups. Commun Math Phys 48:119–130
Liu D, Liu QH, Wu LH, Liu B, Wu J, Lao YM, Li XJ, Gao GF, Ma JC (2009) Website for avian flu information and bioinformatics. Sci China Ser C-Life Sci 52(5):470–473
Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang X (2005) Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 309:1206
Liu M, He S, Walker D, Zhou NN, Perez DR, Mo B (2003) The influenza virus gene pool in a poultry market in south central China. Virology 305:267–275
Longini IM Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA (2005) Containing pandemic influenza at the source. Science 309:1083–1087
Mase M, Tsukamoto K, Imada T, Imai K, Tanimura N, Nakamura K (2005) Characterization of H5N1 influenza A viruses isolated during the 2003–2004 influenza outbreaks in Japan. Virology 332:167–176
Messer JA (2009) A non-equilibrium phase transition in a dissipative forest model. Chaos Soliton Fract 41(5):2456–2462
Mills CE, Robins JM, Lipsitch M (2004) Transmissibility of 1918 pandemic influenza. Nature 432:904–906
Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls JM, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY (2003) Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361(9366):1319–1325
Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y (2004) Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363(9409):617–619
Rao ASRS (2008) Location of the epicenter of avian bird flu might determine the rapidity of its spread in India. Curr Sci 95:313–315
Rohani P, Earn D, Greenfell B (2000) Impact of immunisation on pertussis transmission in England & Wales. Lancet 355:285–286
Rohani P, Breban R, Stallknecht DE, Drake JM (2009) Environmental transmission of low avian influenza viruses and its implications for pathogen invasion. Proc Natl Acad Sci U S A 106(25):10365–10369
Rosenbergova K, Lany P, Pospisil Z, Kubicek O, Celer V, Molinkova D (2009) Quantification of avian influenza virus in tissues of mute swans using TaqMan real time qRT-PCR. Vet Med 54(9):435–443
Seo SH, Peiris M, Webster RG (2002) Protective cross-reactive cellular immunity to lethal A/Goose/Guangdong/1/96-like H5N1 influenza virus is correlated with the proportion of pulmonary CD8+ T cells expressing gamma interferon. J Virol 76:4886–4890
Shortridge KG, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y (1998) Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331–342
Sims LD, Ellis TM, Liu KK, Dyrting K, Wong H, Peiris M (2003) Avian influenza in Hong Kong 1997–2002. Avian Dis 47:832–838
Stech J, Xiong X, Scholtissek C, Webster RG (1999) Independence of evolutionary and mutational rates after transmission of avian influenza viruses to swine. J Virol 73:1878–1884
Sturm-Ramirez KM, Ellis T, Bousfield B, Bissett L, Dyrting K, Rehg JE (2004) Re-emerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol 78:4892–4901
Suri S (2007) Avian influenza (Bird flu), ProQuest CSA LLC discovery guide, pp 1–11. http://www.csa.com/discoveryguides/discoveryguides-main.php
Teyssou R, Rouzic EML (2007) Meningitis epidemics in Africa: a brief overview. Vaccine 25:A3–A7
Upadhyay RK, Kumari N, Sree Hari Rao V (2008) Modeling the spread of bird flu and predicting outbreak diversity. Nonlinear Anal Real World Appl 9(4):1638–1648
Wang SQ, Du QS, Chou KC (2007) Study of drug resistance of chicken influenza A virus (H5N1) from homology-modeled 3D structures of neuraminidases. Biochem Biophys Res Commun 354:634–640
Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y (1992) Evolution and ecology of influenza A viruses. Microbiol Rev 56:152–179
Webster RG, Hulse D (2005) Controlling avian flu at the source. Nature 435:415–417
Yuen K, Chan PKS, Peiris M (1998) Clinical features and rapid viral diagnosis of human disease association with avian influenza A (H5N1) virus. Lancet 351:467–471
Acknowledgment
The research of the first author is supported by a grant from the Foundation for Scientific Research and Technological Innovation (FSRTI)—A Constituent Division of Sri Vadrevu Seshagiri Rao Memorial Charitable Trust, Hyderabad, 500035, India. This work is supported by University Grants Commission, Govt. of India under grant no. F. No. 42-16/2013(SR) to Prof. R.K. Upadhyay.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rao, V.S.H., Upadhyay, R.K. (2013). Modeling the Spread and Outbreak Dynamics of Avian Influenza (H5N1) Virus and Its Possible Control. In: Sree Hari Rao, V., Durvasula, R. (eds) Dynamic Models of Infectious Diseases. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9224-5_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9224-5_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9223-8
Online ISBN: 978-1-4614-9224-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)