Abstract
The histologic diagnosis of lung transplant rejection is based on the assessment of perivascular mononuclear cell inflammation, airway inflammation and fibrosis, and vasculopathic changes. This chapter describes the pathologic features of acute and chronic rejection of the small airways (i.e., lymphocytic and obliterative bronchiolitis). As transbronchial lung biopsy is the mainstay for the assessment of rejection, a brief discussion of some of the limitations of this technique is provided from the pathologist’s perspective. Several important and common entities that can mimic airway rejection are described with practical guidance for distinguishing these potential confounders on transbronchial biopsy. The non-rejection findings that are discussed include the normal biopsy, nonspecific forms of chronic bronchiolitis, cytomegalovirus and pneumocystis pneumonia, bronchiolitis obliterans-organizing pneumonia, and aspiration pneumonia.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Lymphocytic bronchiolitis
- Obliterative bronchiolitis
- Bronchiolitis obliterans syndrome
- Acute rejection
- Chronic rejection
Introduction
Lesions of the small airways are an important manifestation of both acute and chronic rejection of the pulmonary allograft, and two major forms are recognized. The first, lymphocytic bronchiolitis (LB), describes chronic mononuclear cell inflammation of the epithelium and submucosa of the distal small airways (i.e., at the level of and distal to the membranous bronchioles). The second, obliterative (or constrictive) bronchiolitis (OB), refers to partial or complete fibrous scarring of the distal airways and it is often, but not always, pauci-inflammatory. The term “obliterative bronchiolitis” is preferred over the term “bronchiolitis obliterans,” so as not to confuse the former with the much more common bronchiolitis obliterans-organizing pneumonia (BOOP), also termed simply organizing pneumonia (OP), an unrelated disease process with different clinical, radiologic, and pathologic features.
OB is the histologic correlate of the clinically defined bronchiolitis obliterans syndrome (BOS) and remains the gold standard for its definitive diagnosis [1]. While OB is perhaps best known as the central feature of chronic lung rejection, it may also occur in a number of non-transplant settings. Patients with connective tissue diseases, especially rheumatoid arthritis, are perhaps the most commonly affected with OB outside of the transplant setting [2]. In addition, OB is an uncommon complication of various viral infections of the respiratory tract, particularly in children [3, 4], and is also a rare manifestation of medication toxicity (e.g., d-penicillamine) [5] and inhalational injury from various toxins such as ammonia [6], smoke [7], and cocaine [8]. Recently, OB has been described as an occupational lung disorder of microwave popcorn workers (possibly related to diacetyl exposure, a butter-flavoring agent) [9, 10]. OB is also a manifestation of graft-versus-host-disease in allogeneic bone marrow transplant recipients [11]. Interestingly, lesions histologically identical to LB have been observed in some of these conditions [11–13], providing a putative link between LB and OB. Indeed, LB is now widely accepted as not only a bona fide manifestation of acute rejection, but as an important risk factor for developing chronic airway rejection.
The occurrence of OB outside of the transplant setting has contributed to our understanding of the etiology and pathogenesis of this still enigmatic disorder [14–16]. However, post-transplant-related cases remain the most common and increasingly, the best understood examples of OB. As a consequence of the great success of modern immunosuppressive drugs, surgical techniques, and management of infections, with the attendant increase in allograft longevity, OB has emerged as the major long-term obstacle to both graft and patient survival in lung transplantation [17]. This challenge has led to greater emphasis on its early recognition, with the corresponding hope that early treatment can delay or prevent its development [18].
Lymphocytic and obliterative bronchiolitis are part of the formal histologic classification system of lung rejection, developed by the Lung Rejection Study Group of the International Society of Heart and Lung Transplantation (ISHLT). The classification system is published as an ongoing series of working papers in order to maintain up-to-date and standardized nomenclature, and it has undergone two major revisions since the original working formulation was published in 1990 [19–21]. These changes reflect a combination of advances in the field of transplant rejection, experience with the application of the earlier grading schemes, and consensus expert opinion. The current ISHLT Working Formulation [21] recommends histologic assessment of rejection along four lines: Grade A for perivascular inflammation (acute cellular rejection [ACR]), Grade B for airway inflammation (lymphocytic bronchiolitis), Grade C for airway fibrosis (obliterative bronchiolitis), and Grade D for chronic vascular rejection (graft atherosclerosis). ACR is graded on the severity and density of perivascular and interstitial mononuclear cell inflammation in the following manner: A0, no perivascular infiltrates; A1, minimal acute rejection; A2, mild acute rejection; A3, moderate acute rejection; and A4, severe acute rejection. Lymphocytic bronchiolitis is graded as: B0, no airway inflammation; B1R, low-grade airway inflammation; and B2R, high-grade airway inflammation. Constrictive bronchiolitis and chronic vascular rejection are not graded but are designated as being either absent (C0 and D0) or present (C1 and D1). Because bronchoscopy with transbronchial biopsy (TBBx) is the mainstay for assessment of lung rejection, the classification system includes an “ungradeable” score for each parameter, designated by an X after the letter (e.g., AX), if it cannot be assessed in the sample. This reflects the limitations arising from the necessarily limited amount of tissue obtainable with TBBx, the patchiness of the histologic findings in graft rejection, and potential confounding factors, particularly concomitant infection. This classification system can also be applied to larger specimens, such as surgical lung biopsies, explanted allografts, and autopsy material, with the recognition that some findings, especially chronic vascular rejection (Grade D), are relatively uncommon and virtually never identified on TBBx. ACR and graft atherosclerosis will not be further discussed as they are beyond the scope of this chapter. The interested reader will find a good discussion of these topics elsewhere [21–23].
The focus of this chapter is the pathology of the small airways in acute and chronic rejection, with only brief discussion of the potential significance of large airway inflammation (bronchitis). In addition to rejection, the lung transplant patient is at greater risk for a variety of insults that manifest predominantly, both clinically and pathologically, as airway or airway-based abnormalities. Most importantly, this includes opportunistic infections. BOOP and aspiration pneumonia also occur more commonly in transplanted patients and are sometimes overlooked as potential causes of allograft dysfunction. We will also briefly review two entities that may be mistaken for rejection by the pathologist—the normal biopsy and nonspecific forms of chronic bronchiolitis. First however, given the central role that TBBx plays in the management of rejection, we will briefly review the limitations of TBBx, from the perspective of the pathologist.
The Limitations of Transbronchial Lung Biopsy/Adequacy (Fig. 2.1)
Comparing an adequate (a) and a suboptimal (b) transbronchial biopsy (TBBx) at low magnification (hematoxylin and eosin, original magnification ×20). The TBBx in (a) is very generous and contains about eight substantial fragments of alveolated parenchyma. Both large and small airways are well represented (although difficult to discern at this magnification). By comparison, the TBBx in (b) is unsuitable for assessment of rejection. While it consists of about five fragments of tissue, only three are adequately alveolated. However, even these are small, torn, and show significant crush by the forceps. The other fragments are crushed bronchial wall, blood, and exfoliated epithelium, which are not useful for a meaningful histological assessment. The biopsy in (a) obviously provides much more information and is also easier to interpret
There continues to be a spirited debate within the transplant community regarding the utility of the surveillance TBBx (i.e., one that is performed in the asymptomatic patient according to a predetermined schedule) as compared to the clinically indicated TBBx (i.e., one that is performed after the development of signs or symptoms) [24, 25]. Like any procedure TBBx has intrinsic benefits and costs; it is not our intention to enter into the debate regarding the most appropriate role for TBBx. Our focus is instead on the histopathologic findings that facilitate accurate and timely diagnosis in lung transplant patients. Accurate interpretation of TBBx performed for rejection can be challenging for two main reasons. The first, which is common to currently available techniques for retrieving lung tissue with TBBx, stems from the small size and necessarily limited amount of tissue obtainable via the flexible bronchoscope. The second, which is unique to lung transplant patients, is the difficulty in separating bona fide rejection from other processes with a similar appearance. These potential confounders are discussed in greater detail in the latter sections of this chapter.
There is an inherent challenge in interpreting small pieces of tissue that may be crushed or torn. In addition, small pieces of tissue are more difficult to interpret due to problems stemming from oblique (or tangential) sectioning. This is unavoidable in TBBx. However, the problem of limited tissue is ameliorated, to a degree, by the goal for “adequacy” in rejection TBBx. The ISHLT recommends that adequate biopsy tissue sampling consists of at least five pieces of alveolated parenchyma, recognizing that the bronchoscopist may need to submit more pieces than this in order to increase the chances of including histologically assessable bronchioles within the submitted sample. Moreover, the bronchoscopist may need to submit more than five pieces as some, and sometimes all, of the submitted pieces are invariably comprised only of bronchial wall, exfoliated epithelium, and/or blood (see Fig. 2.1). As mentioned above, rejection is a histologically patchy phenomenon. While higher grades of acute rejection are, by definition, more diffuse processes, lower grades of rejection, including grade A2 (the traditional clinical threshold for pulse therapy), can still be very patchy. Moreover, increasing evidence suggests that episodes of even minimal acute rejection or low-grade LB are associated with higher subsequent rates of chronic rejection/BOS [26]. Therefore, obtaining adequate biopsies may be expected to increase the diagnostic yield of TBBx and allow clinicians to appropriately treat an acute rejection episode in patients who would have otherwise gone untreated.
The sensitivity of TBBx for the detection of rejection is less than 100 % even when technically adequate. The yield of TBBx should be distinguished from its sensitivity. The diagnostic yield of TBBx is the percentage of biopsies performed that are “positive” for rejection. Comparing experiences between centers is challenging, in part, because positivity is not uniformly defined. Some have defined as “positive” any biopsy with at least grade A1 rejection, while others define as “positive” biopsies having at least grade A2 rejection, any form or grade of rejection (whether types A, B, or C), and even infection. Diagnostic yield also will vary depending on whether a TBBx is performed for clinical indications or surveillance. Given this variability, diagnostic yield is best understood as an institution-specific parameter and is perhaps most useful as a measure of quality control and improvement. By contrast, sensitivity of rejection TBBx is defined in a standard fashion, which is the fraction of patients with a disease (transplant rejection) who have the disease (rejection) on testing (TBBx). The numerator (i.e., number of patients with rejection on TBBx) is the same whether measuring diagnostic yield or sensitivity, but the denominator is different (yield = number of patients tested; sensitivity = number of patients with rejection). The sensitivity of TBBx varies with the number of pieces obtained. Earlier studies utilizing transplanted animals that were sacrificed [27] showed that five pieces of lung tissue were required to achieve a sensitivity of 92 % for the detection of at least mild rejection. In contrast, a study of 219 TBBx from 54 heart-lung transplant recipients by Scott et al. [28] showed that in the clinical setting 18 samples per procedure may be necessary to have a 95 % confidence of finding rejection.
TBBx is a relatively insensitive method for detecting OB, possessing a sensitivity ranging from 15 % to nearly 40 % [29, 30]. The low sensitivity of TBBx for OB likely stems from three factors: the difficulty in sampling small airways on TBBx, the notoriously patchy nature of OB, and a presumed difficulty of the biopsy forceps in acquiring fibrotic tissue.
The specificity of TBBx for rejection is also less than 100 %, due to the technical challenges of TBBx interpretation and the presence of confounding variables, especially infection. In selected situations, when a TBBx is inadequate or inconclusive and the clinical situation demands a definitive diagnosis, wedge lung biopsy may be a useful option. In a study of 48 open lung biopsies performed on 42 lung transplant patients from an institution performing surveillance TBBx [31], a clinically unsuspected diagnosis was made in 14 (29 %) of the 48 biopsies, all of which resulted in changes to patient treatment. However, this study does not explicitly state the rate of discordance between prior TBBx and wedge biopsy. Nonetheless, it does suggest that wedge lung biopsy can be useful in clinically deteriorating transplant patients for whom TBBx is non-diagnostic.
Lymphocytic Bronchiolitis (Figs. 2.2 and 2.3)
Low-grade lymphocytic bronchiolitis, TBBx (hematoxylin and eosin, original magnification ×200). Mildly to moderately dense lymphocytic inflammation localized predominantly to the bronchiolar submucosa. Lymphocyte crush artifact is prominent, which is common in forceps biopsies. While there are scattered intra-epithelial lymphocytes (arrows), there is no evidence of epithelial cell injury
High-grade lymphocytic bronchiolitis, TBBx (hematoxylin and eosin, original magnification ×100). Very dense lymphoplasmacytic inflammation involving the epithelium and submucosa of a bronchiole (center). The epithelium lining the adjacent larger airway (top) shows no mucosal inflammation. In other levels, the bronchiolar epithelium was necrotic and denuded
Lymphocytic bronchiolitis describes chronic mononuclear cell infiltrates involving the small airways. The current ISHLT Working Formulation subdivides LB into low-grade (B1R) and high-grade (B2R) forms. The “R” in the category designation stands for “revised,” as it reflects a modification of the 1996 working formulation in collapsing the previous four tier grading system (minimal, mild, moderate, severe) into two (low grade and high grade). In addition, there is a category for no airway inflammation (B0) and an ungradeable category (BX) for those cases in which small airways are not present, are not assessable (due to tangential sectioning for example), or for which the inflammation cannot be confidently ascribed to rejection. Indeed, potential confounders that mimic LB have delayed acceptance of utilizing LB as an independent or sole marker for establishing or grading acute rejection. These potential confounders are discussed in detail in the second portion of this chapter (see section on “Non-rejection Findings”).
Low-grade LB is characterized by relatively sparse peribronchiolar lymphocytic inflammation, often in a circumferential or partially circumferential distribution. The lymphocytic infiltrates are localized to the submucosa, which is not expanded, and there should be no evidence of associated epithelial injury. Although the ISHLT definition of low-grade LB restricts the mononuclear infiltrates to the submucosa, scattered intra-epithelial lymphocytes can be observed in otherwise histologically straightforward cases of low-grade LB. For that reason a diagnosis of low-grade LB is appropriate when the infiltrates are not overly dense and localized predominantly to the submucosa.
High-grade LB, by contrast, is characterized by more frequent and increasingly dense peribronchiolar lymphoplasmacytic infiltrates. The lymphocytes may be larger and possess an activated or plasmacytoid appearance. Plasma cells may be present in either low-grade or high-grade LB, but are more numerous in high-grade LB. In contrast to low-grade LB, the denser collections of mononuclear cells (lymphocytes and plasma cells) in high-grade LB tend to infiltrate the basement membrane and epithelium of the bronchioles. In such cases, there will usually be evidence of associated epithelial injury, ranging from epithelial cell apoptosis and necrosis to frank mucosal ulceration. Squamous metaplasia of bronchiolar epithelium may also be present and is testimony to attempts at epithelial regeneration. In addition to mononuclear cells, polymorphonuclear leukocytes, including eosinophils, may be seen with high-grade LB. Neutrophils are usually seen in cases with attendant epithelial necrosis or ulceration. If the luminal infiltrates are frankly purulent, or if there is evidence of distal airspace involvement with neutrophilic inflammation, then acute infection (bronchopneumonia) becomes a more likely explanation for the findings.
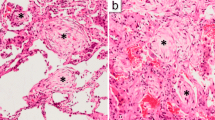
Obliterative (Constrictive) Bronchiolitis (Figs. 2.4 and 2.5)
Obliterative bronchiolitis, TBBx (original magnification ×40). (a) Hematoxylin and eosin-stained section showing collagen fibrosis of a small airway. There is far too much collagen in the wall and submucosa of this bronchiole, lending the wall an excessively thick appearance out of proportion to the caliber of the lumen. The patient showed spirometric evidence of the bronchiolitis obliterans syndrome (BOS). (b) Corresponding trichrome stain. The abnormal and excessive deposition of collagen (blue) within the submucosa and wall of the bronchiole is apparent
Obliterative bronchiolitis, autopsy material. The patient was a 30-year-old female who had received a double lung transplant approximately 8 years earlier for cystic fibrosis. BOS was the cause of death. There was no evidence of acute cellular rejection (ACR). (a) Bronchovascular bundle showing complete occlusive fibrosis of the bronchiole (asterisks) (hematoxylin and eosin, original magnification ×40). Notice the residual fascicles of smooth muscle in the bronchiolar wall (arrows). (b) There is evidence of concomitant lymphocytic bronchiolitis (hematoxylin and eosin, original magnification ×100). This bronchiole shows circumferential submucosal inflammation, which is focally of moderate density. The epithelial sloughing is an artifact of autolysis
OB is the histologic finding of complete or partial bronchiolar fibrosis, whereas BOS is clinically defined as a persistent decline in forced expiratory volume (FEV1) compared to an established post-transplant baseline. While OB is the presumed histologic correlate of BOS, the terms are not interchangeable because some transplant patients develop airflow limitation from other causes [1].
A great deal of basic science, animal model, and clinical research has begun to clarify the pathogenesis of OB and the risk factors predisposing to it [15, 16]. Both ACR and LB are well-established risk factors. Non-immunologic risk factors, including cytomegalovirus (CMV) and non-CMV infection, BOOP, donor age, and graft ischemia time (among others), have also been implicated as risk factors for developing OB/BOS.
Recently, there has been increasing attention on the existence and possible role for antibody-mediated rejection (AMR) as a risk factor for OB/BOS [32]. This stems from the occurrence of OB in patients with no evidence of antecedent ACR [33], evidence of septal capillary injury in cases of otherwise unexplained graft dysfunction [34], complement deposition in capillary endothelium [35], and the uncommon but well-documented occurrence of graft dysfunction in patients who developed donor-specific anti-human leukocyte antigen (anti-HLA) antibodies and capillaritis on biopsy [36]. Despite tantalizing evidence of a possible link between AMR and lung allograft dysfunction there are persistent unresolved questions regarding its diagnosis and significance. Capillaritis has been proposed as a histologic marker of AMR in TBBx but distinguishing capillaritis from simple neutrophil margination (diapedesis) in small specimens is challenging at best. Bronchopneumonia must be rigorously excluded in this setting since neutrophil margination is common in acute infection. In the non-transplant setting necrotizing capillaritis is virtually always associated with clinical and histologic evidence of diffuse alveolar hemorrhage. Immunohistochemical stains for C4d are of limited value given that interpretation is plagued by nonspecific background staining of endothelial cells and elastic tissue. Furthermore, there is a poor correlation between linear C4d staining, the presence of necrotizing capillaritis, and the development of donor-specific HLA alloantibodies [37]. Thus, substantial difficulties remain before AMR can be embraced as a distinct clinicopathologic form of lung rejection.
Collagen fibrosis involving and expanding the bronchiolar submucosa is the histologic hallmark of OB. The fibrosis may be eccentric or concentric and in more advanced lesions results in complete obliteration of the airspace lumen. OB may be more difficult to recognize in the late fibrotic stage as the airways are completely scarred and therefore difficult to recognize. Key to identifying these focal scars as former airways includes the presence of an associated similar-caliber artery or the presence of residual fascicles of smooth muscle within the fibrosis. In most cases, the fibrosis is not accompanied by inflammation but persistence of mononuclear cell inflammation of the sort and character of LB may be noted in some cases. Indeed, LB and OB may coexist. Mucostasis and/or accumulation of foamy, lipid-laden histiocytes within peribronchiolar air spaces may be present as nonspecific markers of small airways dysfunction of any cause. Occasionally these finding are present in the absence of diagnostic small airways changes and are suggestive—but not diagnostic of—bronchiolar pathology.
Large Airway Inflammation/Lymphocytic Bronchitis (Fig. 2.6)
Lymphocytic bronchitis, TBBx (hematoxylin and eosin, original magnification ×200). Dense mononuclear cell inflammation involving a bronchus. Both the wall and epithelium is involved; the latter shows evidence of injury in the form of sloughing and regenerative atypia. There is evidence of small airway involvement as well (lymphocytic bronchiolitis); a tangential portion of an affected bronchiole is seen in the section (arrow). Other fragments (not shown) also showed lymphocytic bronchiolitis. Cultures for microorganisms and other microbiological assays were negative for infection
Lymphocytic bronchiolitis, as the name implies, affects the small airways—that is, the distal-most portions of the conducting bronchioles and the respiratory bronchioles of the allografted lung. The significance of LB with respect to lung rejection is now well established. Occasionally, similar appearing inflammation of the large conducting cartilaginous airways may also occur, with or without associated LB [38]. Unlike LB, much less is known about the significance of isolated large airway inflammation vis-à-vis rejection. Early studies found increased numbers of specialized Leu-7 (CD57)-positive T lymphocytes in the mucosa of donor bronchi with morphologic evidence of airway injury and observed an association between lymphocytic bronchitis and subsequent OB [39–41]. In another study, Yousem and colleagues also found that “chronic inflammation of the bronchi” was associated with subsequent development of OB, with a sensitivity and specificity of 83 % and 100 %, respectively, although the number of cases was very small [42]. Large airway bronchial fibrosis has also been observed in some lung allografts with coexisting OB [43]. These studies suggested that bronchial mucosa may be a target for rejection prompting the use of the combined term “lymphocytic bronchitis/bronchiolitis” (LBB) to refer to the mononuclear cell infiltrates jointly affecting the bronchi and the bronchioles. As is true in small airways, inflammation in large airways is not specific for rejection and is commonly present with clinical (or subclinical) infection, aspiration, chronic obstructive pulmonary disease, and other inhalational injuries. Indeed, the ISHLT working formulation recognizes that large airway inflammation is most commonly associated with infection and aspiration and does not currently identify or grade “lymphocytic bronchitis” as such [21]. Bronchiectasis has also been described in lung transplant patients with BOS, although it is not known if this is a consequence of infection, rejection, ischemic injury, or some combination of these factors [44].
The significance of lymphocytic bronchitis in a TBBx depends upon not only the morphologic features but also the clinical context. To help distinguish lymphocytic bronchitis from nonspecific forms of chronic bronchitis the term should be limited to cases in which dense collections of lymphocytes are confined to the bronchial submucosa and submucosal glands, often infiltrating into the bronchial epithelium. With more intense degrees of inflammation, greater numbers of transformed lymphocytes, immunoblasts, and even eosinophils are present, and there may be evidence of epithelial injury including apoptosis, squamous metaplasia, or ulceration. Neutrophils should not be abundant and there should not be evidence of viral cytopathic change or aspiration, features which would point to another etiology. When narrowly defined in this way lymphocytic bronchitis is not a common finding and, when present, is often seen in combination with other findings typical of acute rejection, usually LB. Such cases should be graded conventionally as per ISHLT guidelines, with or without mention of the presence of lymphocytic bronchitis, since in any event clinical decision making will be based on the formal “A-B-C” rejection grade. Lymphocytic bronchitis is uncommon as a truly isolated finding, and when present without other corroborative histologic support for a diagnosis of rejection its significance is uncertain.
Non-rejection Findings
Normal (Bronchus-Associated Lymphoid Tissue) (Fig. 2.7)
Bronchus-associated lymphoid tissue, wedge biopsy (hematoxylin and eosin, original magnification ×200). BALT, a normal finding, comprises mucosal lymphoid aggregates associated with large and/or small airways. The aggregates are comprised of well-circumscribed subepithelial primary or secondary lymphoid follicles. In the small airways, as seen here, the lymphocytes may focally percolate among the epithelial cells (“lymphoepithelium,” arrow). It may sometimes be difficult to distinguish BALT from bronchiolitis (of any cause) on TBBx, particularly when the sample is very small, fragmented, or crushed
The airways, as in other non-sterile mucosal sites with a more-or-less constant exposure to the external environment, possess a mucosa-associated lymphoid tissue (MALT tissue) specifically referred to as bronchus-associated lymphoid tissue (BALT). In the large airways, these comprise circumscribed submucosal aggregates (primary follicles) of lymphocytes. They are usually not very prominent, unless there has been antigenic stimulation, in which case there may be BALT hyperplasia which may be associated with germinal center formation (secondary follicles). The circumscription and submucosal localization of BALT follicular aggregates is not likely to be confused with lymphocytic bronchitis and bronchiolitis. However, in the intermediate and small airways, the lymphocytes may extend into the overlying epithelium (“lymphoepithelium”), which is focally attenuated as it is in other MALT sites. It is important not to confuse this normal finding with LB (or with any other pathology). The key features distinguishing BALT and lymphoepithelium from LB are that the lymphoid infiltrates of the latter are denser, are not circumscribed, and do not form primary or secondary follicles; establishing this may require assessment of multiple consecutive tissue levels. Furthermore, LB may be associated with epithelial damage including epithelial cell necrosis, mucosal ulceration, and squamous metaplasia, particularly when high grade (B2R). Lastly, LB is often associated with the perivascular lymphoid infiltrates of ACR.
Nonspecific Chronic Bronchiolitis
Chronic bronchiolitis is a histopathologic term referring to chronic inflammation involving bronchiolar and peribronchiolar interstitium with or without fibrosis [45]. Chronic bronchiolitis is a nonspecific finding; its significance is defined by the histopathologic and clinical context [46]. For example, chronic bronchiolitis is a common finding in other primary pathologic processes, such as hypersensitivity pneumonia. In hypersensitivity pneumonia, chronic bronchiolitis is only one component of a unique combination of equally nonspecific findings that is characteristic only when present collectively. Chronic bronchiolitis is uncommon as an isolated primary pathologic process and occurs in surprisingly heterogeneous clinical contexts. In smokers with evidence of obstructive airways disease, chronic bronchiolitis corresponds to the small airways disease thought to account for airflow limitation in patients with emphysema and chronic bronchitis [47]. Occasional unexplained chronic bronchiolitis occurs in nonsmokers with airflow limitation who lack other features of emphysema, chronic bronchitis, or asthma (i.e., idiopathic small airways disease). Chronic bronchiolitis does not by itself predict for physiologically significant obstructive airways disease, however, and in some patients may actually be affiliated with evidence of restrictive lung disease.
Given the nonspecific nature of chronic bronchiolitis and the wide range of potential causes and associations, attributing bronchiolitis to rejection in transplant patients requires careful integration of not only histopathologic but also clinical, physiologic, and radiologic data.
Opportunistic Infection
Infectious complications are a major obstacle to both short-term and long-term survival in lung transplantation. Non-CMV infections are the leading cause of morbidity and mortality in the first year status post-transplantation, and remain the second leading cause of mortality thereafter, preceded only by BOS [17]. Pneumonia, particularly bacterial pneumonia, is the most common infection affecting lung transplant patients, especially in the early post-transplant period, although mycobacterial, viral, and fungal pneumonia all occur at an increased frequency in lung transplant patients [48]. For the pathologist, the diagnosis of acute bronchopneumonia due to pyogenic bacteria or granulomatous infection is generally straightforward and not likely to be confused with acute rejection; the former entities are characterized by suppurative or granulomatous inflammation involving the airspaces, while acute rejection is typified by mononuclear/lymphocytic inflammation in the perivascular and peribronchiolar interstitium.
Certain infectious agents produce a cellular interstitial pneumonia that is more likely to be confused with acute rejection. In particular, two important opportunistic pathogens, CMV and Pneumocystis jirovecii, cause an infectious pneumonia that may show prominent chronic interstitial inflammation (i.e., chronic interstitial pneumonia) that closely resembles acute rejection [49, 50]. In a study of CMV and pneumocystis pneumonia diagnosed by open lung biopsy and TBBx, Tazelaar [50] noted perivascular lymphocytic infiltrates similar to those seen in acute rejection in 42 % of CMV cases and 21 % of pneumocystis cases. Such results reiterate the need for the pathologist to at least consider the possibility of infection in every transplant TBBx and to rigorously exclude—or include—infection with ancillary special stains in selected cases. A TBBx diagnosis of infection that includes perivascular lymphoid infiltrates does not preclude the possibility of concomitant rejection, however, and should be regarded as indeterminate (“AXBX”) in this regard. If clinically warranted, a subsequent TBBx following appropriate antimicrobial treatment may be more helpful in evaluating for rejection without the confounding effects of infection. This serves as a reminder that the ultimate diagnosis in any individual patient should be the result of integration with all available clinical data, including those from microbiologic and serologic studies.
Among non-alloimmune risk factors for the development of OB/BOS, pulmonary infection due to CMV has been the most extensively studied, with relatively fewer reports analyzing non-CMV viruses, bacteria, and fungi including pneumocystis [14, 16]. Bacterial and pneumocystis pneumonia have not been clearly shown to be significant risk factors for OB/BOS, while studies assessing the significance of CMV pneumonia on the development of OB/BOS have shown inconsistent results [14, 16]. At this time, pulmonary infections, in general, and viral respiratory pathogens, in particular, are considered to be possible risk factors for OB/BOS, perhaps by potentiating the effects of acute rejection.
Cytomegalovirus Pneumonia (Fig. 2.8)
Cytomegalovirus (CMV) pneumonia, TBBx. (a) On low power, there is a dense predominantly chronic inflammatory infiltrate involving the bronchus and subjacent alveolar tissue(hematoxylin and eosin, original magnification ×100). Such an appearance is reminiscent of high-grade ACR with lymphocytic bronchitis/bronchiolitis. (b) On higher magnification, an endothelial cell with CMV viral cytopathic change is seen (arrow) (hematoxylin and eosin, original magnification ×400). This comprises nucleomegaly and cytomegaly and basophilic ground glass nuclear inclusions. There may also be basophilic intracytoplasmic granules, although these are somewhat difficult to discern even at this magnification. An immunohistochemical stain for CMV was also positive (not shown). Note the marked lymphohistiocytic inflammation
The key to the diagnosis of CMV pneumonia is the recognition of characteristic viral cytopathic changes caused by CMV infection, of which there are three—cytomegaly, nuclear inclusions, and cytoplasmic inclusions. Cellular and nuclear enlargement (cytomegaly) is perhaps the most easily recognizable alteration. The intranuclear inclusions consist of centrally placed amorphous basophilic inclusions, usually with a clear halo separating them from the nuclear membrane. The cytoplasmic inclusions, which are not seen in every infected cell, are also basophilic and coarsely granular. The latter often stain positively with the Gomori methenamine silver (GMS) method. These viral cytopathic changes can affect virtually any cell, including pneumocytes, interstitial cells, and endothelial cells. While some cases may show numerous CMV virocytes, other cases may show only a few or rare infected cells, particularly in the limited samples that TBBx provides. An immunohistochemical stain for CMV is widely available and can be very helpful in confirming the diagnosis, especially in subtle cases. In addition to the characteristic altered cells, CMV pneumonia typically elicits a predominantly chronic inflammatory cell reaction involving the interstitium and the airways that may be nearly indistinguishable from ACR and LB. In more severe cases, it may also cause diffuse alveolar damage (DAD) and/or fibrinous airspace exudate.
As stated above, unless the viral cytopathic changes are recognized, the case is likely to be misdiagnosed as acute rejection. The viral changes caused by CMV must be distinguished from those due to herpes simplex virus (HSV). HSV infection does not result in significant cytomegaly, nor does it cause intracytoplasmic inclusions. In addition, HSV infection produces ground glass intranuclear inclusions that are usually prominently eosinophilic and with a margin of peripherally condensed chromatin.
Pneumocystis jirovecii Pneumonia (Fig. 2.9)
Pneumocystis jirovecii pneumonia, TBBx. (a) This photomicrograph demonstrates frothy, eosinophilic alveolar exudates, the most helpful and characteristic feature of Pneumocystis pneumonia (hematoxylin and eosin, original magnification ×200). (b) These exudates have a distinctive microcystic appearance at high magnification (hematoxylin and eosin, original magnification ×400). (c) Silver stains, such as the Gomori methenamine silver (GMS) stain, demonstrate the yeast forms, which are 4–6 μm in diameter and helmet-shaped, crescentic, or spherical (GMS, original magnification ×600). Note the internal dot-like enhancement inside the cysts (arrow), a feature which helps distinguish Pneumocystis from Histoplasma spp. yeast forms
There are a number of histologic changes that can be seen in pneumocystis pneumonia. The classic change is the presence of an eosinophilic “frothy” alveolar exudate on hematoxylin and eosin (H&E) staining. On higher power, this exudate possesses a honeycomb-like or microcystic appearance, representing numerous organism cysts and it is pathognomonic for the disease. This frothy exudate may be associated with features of DAD including hyaline membranes. Granulomatous inflammation—necrotizing, non-necrotizing, or both—is an uncommon manifestation of pneumocystis pneumonia that is often associated with lymphocytic inflammation and clusters of histiocytes. Other less common changes include areas of necrosis, calcification, and a pulmonary alveolar proteinosis-like reaction.
TBBx is a sensitive technique for the detection of pneumocystis pneumonia. If the characteristic frothy eosinophilic alveolar exudates are present, then the diagnosis is straightforward and can be made even in the absence of special stains. Occasionally, however, only hyaline membranes, a chronic interstitial pneumonia, or granulomas are present. For that reason, it is important to maintain a low threshold for performing special stains, especially stains such as a GMS stain that highlight pneumocystis organisms.
Bronchiolitis Obliterans-Organizing Pneumonia (Fig. 2.10)
Bronchiolitis obliterans-organizing pneumonia, TBBx (hematoxylin and eosin, original magnification ×100). Fibromyxoid plugs of spindled fibroblasts and myofibroblasts are present within the airspaces and lumens of distal airways. When encountering a TBBx with BOOP, the pathologist should search for more specific features that might suggest an underlying etiology, such as evidence of acute infection, viral changes, granulomas, or aspirated foreign material. Notice the presence of an associated cellular chronic interstitial pneumonia, a common associated finding in BOOP (of any cause), and one that should be distinguished from the perivascular mononuclear cell infiltrates of ACR
Bronchiolitis obliterans-organizing pneumonia (BOOP), also termed organizing pneumonia (OP), is a nonspecific manifestation of acute lung injury. As such, it can be caused by or associated with a wide variety of insults and conditions, including infectious pneumonia, medications, aspiration of gastric contents, radiation, or connective tissue disease [51]. The etiology is usually not apparent on the basis of the histologic findings alone. BOOP may also be seen as a nonspecific secondary change in other primary processes. Idiopathic BOOP, also termed cryptogenic OP or COP, refers to a distinct syndrome of unknown cause with characteristic clinical and radiographic features and BOOP as an isolated finding on lung biopsy [52]. Spontaneous remission may occur, and in those patient requiring treatment it tends to be a steroid-responsive disease, although relapses are common. These features are in contrast to OB, which is typically insidious and progressive and not marked by relapses or remissions. BOOP is a fairly common finding in rejection biopsies [53], reemphasizing the importance of its distinction from OB by the reviewing pathologist. Indeed, in an earlier review of organizing pneumonia-like reactions in allograft biopsies, Yousem and colleagues described BOOP as most commonly occurring in the setting of acute rejection [53]. Several groups have also found BOOP to be a risk factor for OB/BOS [54, 55]. As such, BOOP has been proposed to be included in the histologic classification of lung rejection [56], although this has not been adopted.
BOOP is characterized by fusiform proliferations of spindled fibroblastic and myofibroblastic cells set within a pale-staining myxoid matrix containing abundant mucopolysaccharides (ground substance), a combination of findings sometimes described as fibromyxoid plugs of “young” fibrosis. A key feature defining BOOP is the localization of these fibromyxoid plugs to the lumens of the distal bronchioles (“bronchiolitis obliterans”) and alveolar airspaces and ducts (“organizing pneumonia”). This distribution accounts for its typical whorled and serpentine appearance. Involvement of the bronchiolar lumens causes small airway dysfunction, which in turn results in a variably prominent accumulation of foamy macrophages, sometimes referred to as endogenous lipoid pneumonia. BOOP may be accompanied by abundant airspace fibrin, lending an eosinophilic appearance to the process. Associated inflammation can be highly variable, from negligible to dense infiltrates, and is usually comprised of chronic inflammatory cells, mostly lymphocytes and plasma cells. The inflammatory cells can be found within the fibromyxoid tissue or alveolar septal walls or both. However, if alveolar septal and perivascular mononuclear infiltrates are prominent, then high-grade ACR should be strongly considered as the underlying etiology. Neutrophils and histiocytes may also be found, but if prominent, an infectious etiology should be suspected and the use of special stains for microorganisms may be helpful in further evaluating for that possibility.
The organizing phase of DAD may be indistinguishable from BOOP in small biopsies. BOOP can usually be distinguished by the intraluminal localization of the fibroblastic plugs and the absence of hyaline membranes but these helpful clues are not always easily discerned in TBBx. DAD typically occurs in the setting of the adult respiratory distress syndrome (ARDS) and for that reason can usually be separated from BOOP by correlating with the patient’s clinical status in histologically challenging cases. BOOP is usually easily distinguishable from OB, even in small biopsies, as both the location (airspaces in the former, submucosa in the latter) and constitutive elements (fibromyxoid tissue in the former, collagen fibrosis in the latter) are distinctly different.
Aspiration Pneumonia (Fig. 2.11)
Aspiration pneumonia, TBBx. (a) Intermediate magnification photomicrograph showing BOOP with granulomatous inflammation (hematoxylin and eosin, original magnification ×200). Multinucleated giant cells are engulfing aspirated exogenous substances. The amorphous pale-staining material within the upper giant cell (arrow) is partially digested foodstuff while the birefringent, cracked, crystalline material in the lower giant cell (asterisk) is microcrystalline cellulose, a common inorganic filler utilized in oral medications. (b) Polarized light microscopy can be helpful in identifying and/or confirming polarizable substances in suspected cases of aspiration (hematoxylin and eosin, original magnification ×600). Certain crystalline fillers, such as microcrystalline cellulose (as seen here) are strongly polarizable. Plant cell walls from aspirated foods vary greatly in their strength of polarization
Patients who have undergone lung transplantation are at a significantly increased risk for gastroesophageal reflux and aspiration [57–59], possibly due to impaired cough reflex and mucociliary clearance mechanisms. While massive acute aspiration is not a clinically occult condition, chronic, low-level episodes of repeated aspiration pose a more difficult diagnostic challenge; in fact, chronic aspiration is often clinically unsuspected [60, 61]. Chronic gastroesophageal reflux and aspiration have been implicated as non-alloimmune risk factors for the development of OB/BOS [62], and anti-reflux therapy utilizing medical (macrolide antibiotics) and surgical (gastric fundoplication) modalities has resulted in improved lung function in several studies [63–66]. Thus, aspiration is a treatable cause of pulmonary allograft dysfunction and it is a diagnosis the pathologist is often in a unique position to make.
The morphologic features of particulate aspiration are sufficiently unique that the diagnosis can often be made on TBBx. Aspiration pneumonia is characterized by airway-centered granulomatous inflammation that is often necrotizing. The granulomas typically elicit an associated BOOP response, which is often quite prominent and is sometimes the dominant finding. Acute and chronic bronchitis and bronchiolitis are a nearly constant finding and thus the pathologist must take care before ascribing bronchiolitis to rejection or to infection. The defining feature of aspiration pneumonia is the presence of exogenous aspirated foreign material, either in an extracellular location or within giant cells or both. The aspirated material is of two major kinds—foodstuffs and inorganic crystalline “fillers” derived from oral medications; the presence of either substance in the appropriate histologic context is diagnostic. The foodstuffs have a varying appearance depending on the age of the process. They include recognizable skeletal muscle and plant cell walls derived from consumed meats and vegetables, respectively; the latter may be refractile and either weakly or strongly birefringent on polarized microscopy. Older organic material tends to have a pale, amorphous eosinophilic appearance, and is more difficult to recognize. The most common inorganic fillers include microcrystalline cellulose, which is strongly birefringent, and crospovidone, which has an amorphous densely basophilic appearance. These exogenous compounds must not be confused with various endogenous materials that can be found within giant cells, including blue bodies, asteroid bodies, and birefringent calcium salts. As mentioned above, the granulomas in aspiration sometimes show central suppurative necrosis, wherein the giant cells surround pockets of neutrophils. The latter feature, while nonspecific (as it can be seen with certain infections, Wegener granulomatosis, and rheumatoid nodules) is not common and is therefore a potential clue to the diagnosis. If suppurative granulomas are present in a TBBx, this should prompt the pathologist to search carefully for any associated exogenous aspirated substances. Occasionally, no aspirated material can be found, a problem more common in small biopsies, and a confident diagnosis of aspiration pneumonia may not be possible. In immunocompromised patients the differential diagnosis for otherwise unexplained granulomatous inflammation includes mainly opportunistic infections and should prompt appropriate special stains and microbiological assays. Organisms that may cause suppurative granulomatous inflammation resembling that seen in aspiration include, most commonly, Nocardia, Actinomyces, and Blastomyces species.
References
Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310.
Gauhar UA, Gaffo AL, Alarcon GS. Pulmonary manifestations of rheumatoid arthritis. Semin Respir Crit Care Med. 2007;28(4):430–40.
Sung RYT, Chan RCK, Tam JS, Cheng AFB, Murray HGS. Epidemiology and etiology of acute bronchiolitis in Hong-Kong infants. Epidemiol Infect. 1992;108(1):147–54.
Aguerre V, Castanos C, Pena HG, Grenoville M, Murtagh P. Postinfectious bronchiolitis obliterans in children: clinical and pulmonary function findings. Pediatr Pulmonol. 2010;45(12):1180–5.
Takayama S, Ogawa T, Tominaga S, Yasui M, Ohno S, Ohkochi M, et al. [Penicillamine-induced bronchiolitis obliterans diagnosed by transbronchial lung biopsy]. Nihon Kokyuki Gakkai Zasshi. 2006;44(2):128–33.
Price SK, Hughes JE, Morrison SC, Potgieter PD. Fatal ammonia inhalation. A case report with autopsy findings. S Afr Med J. 1983;64(24):952–5.
Tasaka S, Kanazawa M, Mori M, Fujishima S, Ishizaka A, Yamasawa F, et al. Long-term course of bronchiectasis and bronchiolitis obliterans as late complication of smoke inhalation. Respiration. 1995;62(1):40–2.
Ettinger NA, Albin RJ. A review of the respiratory effects of smoking cocaine. Am J Med. 1989;87(6):664–8.
Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347(5):330–8.
Harber P, Saechao K, Boomus C. Diacetyl-induced lung disease. Toxicol Rev. 2006;25(4):261–72.
Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone-marrow transplant recipients. Hum Pathol. 1995;26(6):668–75.
Workman DL, Clancy Jr J. Interstitial pneumonitis and lymphocytic bronchiolitis/bronchitis as a direct result of acute lethal graft-versus-host disease duplicate the histopathology of lung allograft rejection. Transplantation. 1994;58(2):207–13.
Boehler A, Chamberlain D, Kesten S, Slutsky AS, Liu MY, Keshavjee S. Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation. 1997;64(2):311–7.
Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21(2):271–81.
Neuringer IP, Chalermskulrat W, Aris R. Obliterative bronchiolitis or chronic lung allograft rejection: a basic science review. J Heart Lung Transplant. 2005;24(1):3–19.
Scott AIR, Sharples LD, Stewart S. Bronchiolitis obliterans syndrome—risk factors and therapeutic strategies. Drugs. 2005;65(6):761–71.
Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult lung and heart-lung transplant report-2011. J Heart Lung Transplant. 2011;30(10):1104–22.
Van Muylem A, Knoop C, Estenne M. Early detection of chronic pulmonary allograft dysfunction by exhaled biomarkers. Am J Respir Crit Care Med. 2007;175(7):731–6.
Yousem SA, Berry GJ, Brunt EM, Chamberlain D, Hruban RH, Sibley RK, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection—Lung Rejection Study-Group. J Heart Transplant. 1990;9(6):593–601.
Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15.
Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–42.
Radio S, Wood S, Wilson J, Lin H, Winters G, McManus B. Allograft vascular disease: comparison of heart and other grafted organs. Transplant Proc. 1996;28(1):496–9.
Yousem SA, Paradis IL, Dauber JH, Zeevi A, Duquesnoy RJ, Dalcol R, et al. Pulmonary arteriosclerosis in long-term human heart-lung transplant recipients. Transplantation. 1989;47(3):564–9.
Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med. 2010;31(2):208–21.
Sandrini A, Glanville AR. The controversial role of surveillance bronchoscopy after lung transplantation. Curr Opin Organ Transplant. 2009;14(5):494–8.
Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004;170(9):1022–6.
Tazelaar HD, Nilsson FN, Rinaldi M, Murtaugh P, McDougall JC, McGregor CG. The sensitivity of transbronchial biopsy for the diagnosis of acute lung rejection. J Thorac Cardiovasc Surg. 1993;105(4):674–8.
Scott JP, Fradet G, Smyth RL, Mullins P, Pratt A, Clelland CA, et al. Prospective study of transbronchial biopsies in the management of heart-lung and single lung-transplant patients. J Heart Lung Transplant. 1991;10(5):626–37.
Kramer MR, Stoehr C, Whang JL, Berry GJ, Sibley R, Marshall SE, et al. The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: low yield of transbronchial lung biopsy. J Heart Lung Transplant. 1993;12(4):675–81 [Research Support, U.S. Gov’t, P.H.S.].
Cagle PT, Brown RW, Frost A, Kellar C, Yousem SA. Diagnosis of chronic lung transplant rejection by transbronchial biopsy. Mod Pathol. 1995;8(2):137–42 [Comparative Study].
Weill D, McGiffin DC, Zorn GL, Alexander CB, Early LJ, Kirklin JK, et al. The utility of open lung biopsy following lung transplantation. J Heart Lung Transplant. 2000;19(9):852–7.
Glanville AR. Antibody-mediated rejection in lung transplantation: myth or reality? J Heart Lung Transplant. 2010;29(4):395–400.
Magro CM, Abbas AE, Seilstad K, Pope-Harman AL, Nadasdy T, Ross P. C3d and the septal microvasculature as a predictor of chronic lung allograft dysfunction. Hum Immunol. 2006;67(4–5):274–83.
Badesch DB, Zamora M, Fullerton D, Weill D, Tuder R, Grover F, et al. Pulmonary capillaritis: a possible histologic form of acute pulmonary allograft rejection. J Heart Lung Transplant. 1998;17(4):415–22.
Magro CM, Harman AP, Klinger D, Orosz C, Adams P, Waldman J, et al. Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am J Transplant. 2003;3(9):1143–54.
Morrell MR, Patterson GA, Trulock EP, Hachem RR. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2009;28(1):96–100.
Chantranuwat C, Qiao JH, Kobashigawa J, Hong L, Shintaku P, Fishbein MC. Immunoperoxidase staining for C4d on paraffin-embedded tissue in cardiac allograft endomyocardial biopsies: comparison to frozen tissue immunofluorescence. Appl Immunohistochem Mol Morphol. 2004;12(2):166–71.
Yousem SA. Lymphocytic bronchitis bronchiolitis in lung allograft recipients. Am J Surg Pathol. 1993;17(5):491–6.
Beschorner WE, Saral R, Hutchins GM, Tutschka PJ, Santos GW. Lymphocytic bronchitis associated with graft-versus-host disease in recipients of bone-marrow transplants. N Engl J Med. 1978;299(19):1030–6.
Hruban RH, Beschorner WE, Gupta PK, Baumgartner WA, Achuff SC, Traill TA, et al. Diagnosis of rejection of lung allografts. N Engl J Med. 1988;318(17):1129.
Hruban RH, Beschorner WE, Baumgartner WA, Achuff SC, Traill TA, Marsh BR, et al. Diagnosis of lung allograft rejection by bronchial intraepithelial Leu-7 positive T lymphocytes. J Thorac Cardiovasc Surg. 1988;96(6):939–46.
Yousem SA, Paradis IL, Dauber JA, Zeevi A, Rabinowich H, Duquesnoy R, et al. Large airway inflammation in heart-lung transplant recipients—its significance and prognostic implications. Transplantation. 1990;49(3):654–6.
Akindipe O, Fernandez-Bussy S, Jantz M, Lu L, Deem A, Swafford W, et al. Obliterative bronchiolitis in lung allografts removed at retransplant for intractable airway problems. Respirology. 2009;14(4):601–5.
Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation—a review. Chest. 1998;114(5):1411–26.
Visscher DW, Myers JL. Bronchiolitis: the pathologist’s perspective. Proc Am Thorac Soc. 2006;3(1):41–7.
Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168(11):1277–92.
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53.
Remund KF, Best M, Egan JJ. Infections relevant to lung transplantation. Proc Am Thorac Soc. 2009;6(1):94–100.
Nakhleh RE, Bolman RM, Henke CA, Hertz MI. Lung-transplant pathology—a comparative-study of pulmonary acute rejection and cytomegaloviral infection. Am J Surg Pathol. 1991;15(12):1197–201.
Tazelaar HD. Perivascular inflammation in pulmonary infections: implications for the diagnosis of lung rejection. J Heart Lung Transplant. 1991;10(3):437–41.
Katzenstein A-LA, Askin FB. Katzenstein and Askin’s surgical pathology of non-neoplastic lung disease. 4th ed. Philadelphia, PA: Elsevier Saunders; 2006.
Lohr RH, Boland BJ, Douglas WW, Dockrell DH, Colby TV, Swensen SJ, et al. Organizing pneumonia. Features and prognosis of cryptogenic, secondary, and focal variants. Arch Intern Med. 1997;157(12):1323–9.
Yousem SA, Duncan SR, Griffith BP. Interstitial and airspace granulation-tissue reactions in lung-transplant recipients. Am J Surg Pathol. 1992;16(9):877–84.
Milne DS, Gascoigne AD, Ashcroft T, Sviland L, Malcolm AJ, Corris PA. Organizing pneumonia following pulmonary transplantation and the development of obliterative Bronchiolitis. Transplantation. 1994;57(12):1757–62.
Girgis RE, Tu IP, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15(12):1200–8.
Gabbay E, Dark JH, Ashcroft T, Corris PA. Cryptogenic organizing pneumonia is an important cause of graft dysfunction and should be included in the classification of pulmonary allograft rejection. J Heart Lung Transplant. 1998;17(2):230–1.
Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124(5):1689–93.
Blondeau K, Merters V, Vanaudenaerde BA, Verleder GM, Van Raemdonck DE, Sifrim D, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31(4):707–13.
D’Ovidio F, Keshavjee S. Gastroesophageal reflux and lung transplantation. Dis Esophagus. 2006;19(5):315–20.
Mukhopadhyay S, Katzenstein A-LA. Pulmonary disease due to aspiration of food and other particulate matter: a clinicopathologic study of 59 cases diagnosed on biopsy or resection specimens. Am J Surg Pathol. 2007;31(5):752–9.
Barnes TW, Vassallo R, Tazelaar HD, Hartman TE, Ryu JH. Diffuse bronchiolar disease due to chronic occult aspiration. Mayo Clin Proc. 2006;81(2):172–6.
Rinaldi M, Martinelli L, Volpato G, Pederzolli C, Silvestri M, Pederzolli N, et al. Gastroesophageal reflux as cause of obliterative bronchiolitis—a case-report. Transplant Proc. 1995;27(3):2006–7.
Cantu E, Appel JZ, Hartwig MG, Woreta H, Green C, Messier R, et al. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78(4):1142–51.
Davis RD, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125(3):533–42.
Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;77(9):1465–7.
Yates B, Murphy DM, Forrest IA, Ward C, Rutherford RM, Fisher AJ, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172(6):772–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lagstein, A., Myers, J. (2013). Airway Pathology in Lung Transplants. In: Meyer, K., Glanville, A. (eds) Bronchiolitis Obliterans Syndrome in Lung Transplantation. Respiratory Medicine, vol 8. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7636-8_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7636-8_2
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7635-1
Online ISBN: 978-1-4614-7636-8
eBook Packages: MedicineMedicine (R0)