Abstract
A single encounter of a response together with a stimulus results in short-lived binding between the stimulus and the response. A repetition of any part of such a stimulus–response episode can then retrieve the whole episode, including the response. Recent findings have shown that similar binding is also possible between two successive but independently planned manual responses, indicating that binding processes also play a role in the coordination of action sequences. Action coordination in everyday life often includes alternation between different effector sets. Yet switching effectors has been shown to result in very clear partitioning of actions. Thus, it is unclear whether responses carried out via different effector sets (feet and hands) are as easily integrated as responses via a single effector set (hands). In two experiments, we investigated whether response–response integration is possible across effector-set switches, and compared the binding effects across effector sets to those within one effector set. In a prime–probe design, participants executed two responses at the prime and the probe—the first via their hands and the second via their feet (Exp. 1), or the first via either hands or feet and the second via hands (Exp. 2). The data from both experiments indicated binding between responses, even if the actions were carried out via different effector sets. However, bindings between responses that were carried out via different effector sets were weaker than bindings between responses via a single effector set. We concluded that binding constitutes a main function of action sequences in human behavior.
Similar content being viewed by others
Integration and retrieval processes are understood to play a core role in action control. Responding to a stimulus leads to integration of stimulus and response features in a representation called an event file (Hommel, Müsseler, Aschersleben, & Prinz, 2001) or instance (Logan, 1988; Schmidt, De Houwer, & Rothermund, 2016), so that upon repetition of any of the features, the others are retrieved, influencing current performance in what is known as binding effects (Frings et al., in press; Henson, Eckstein, Waszak, Frings, & Horner, 2014): After stimulus–response (SR) integration, repeating any part of the episode can trigger retrieval of the entire episode, including response features. If a retrieved response matches the currently required one, performance improves. Yet performance can also be impaired, if the retrieved and required responses differ.
Recently, it has been shown that not only SR binding in the representation of individual actions, but also binding between successive responses, plays a role in action control (RR bindings; Moeller & Frings, 2019a, b). Participants executed pairs of responses, in which planning of the second response was possible only after the first response had been executed. Whether these responses were integrated was then measured in the two successive responses: If the first of the successive responses was repeated from before, it was assumed to retrieve the other response, influencing the second of the successive responses. Similar to the mechanisms known from SR binding, planning and carrying out two independent responses led to the integration of these responses (independent of the stimuli), so that repeating one of them retrieved the other response. This finding substantially extends the situations in which binding processes contribute to successful action control. Apparently, not only are individual responses integrated with stimulus features, but binding processes also support the coordination of action sequences.
Until now, the responses that have been shown to become integrated were invariably carried out with the same effector set, namely the hands. By contrast, everyday action control obviously includes the coordination of various effectors. Notably, this difference might challenge the claim that binding supports the coordination of actions on a regular basis. Actions that are carried out via different effector sets seem to be particularly separated in their representations (e.g., Philipp & Koch, 2005, 2011; Stoet & Hommel, 1999). For example, Eimer, Schubö, and Schlaghecken (2002) found response inhibition effects in a masked-priming paradigm only if effector sets (hands vs. feet) repeated between responses, but not if one response was executed via the hands and the other via the feet. In addition, Braem, Verguts, and Notebeart (2011) found better discrimination between tasks if participants used their hands to respond to one task and their feet to respond to the other, as compared to when participants used hand responses in both tasks. Finally, Moeller, Hommel, and Frings (2015) had participants respond to visually presented stimuli either via keypresses with fingers or via pedal presses with their feet. Individual responses led to SR integration and response retrieval at stimulus repetition. However, the binding effects were diminished if participants switched effector sets between SR integration and retrieval. Note that the mentioned studies investigated individual responses. These seem to be represented as particularly separate if they are executed via different effector sets. Shifting our focus to pairs of individual responses and the question of whether these can be integrated with each other, these studies give us reason to assume that responses carried out with different effector sets are separated to a degree that prevents the integration of these responses.
Yet, from a slightly different angle, there is no reason to assume that a switch of effector set would prevent RR-binding effects. Binding effects always rely on two different processes (Frings et al., in press). In a first step, features need to be integrated with each other before repetition of one of the features can then elicit retrieval of the other. In the study by Moeller et al. (2015), upon first execution of a single response, SR integration was assumed, and upon execution of a single second response, response retrieval due to the repetition of stimulus features was measured. Importantly, effector sets (hands vs. feet) were independently assigned to the responses, so that effector-set switches always occurred after integration, but before retrieval would have taken place. Therefore, it is likely that the lack of binding effects was due to the fact that integration and retrieval were separated by an effector-set switch, and that this separation hindered retrieval of the integrated response. An effector-set change during the integration of two separate responses (and again across retrieval) might not affect binding effects to the same extent. Hence, integration of responses that are carried out with different effector sets might be possible.
In addition, it has been suggested that action planning is effector-unspecific. Responses seem to be represented in terms of their action goals rather than specific motor programs (e.g., Eder, Müsseler, & Hommel, 2012; Prinz, 1997; see also Rosenbaum, 1980; R. A. Schmidt, 1975; Wright, 1990). That is, integration of individual actions might take place on a level of goal representation and not rely on both actions being executed via the same effector set.
In summary, so far it is unclear whether the partitioning of actions still allows for bindings between individual responses if they are executed via different effector sets. Thus, the present study was designed to test whether successively planned and executed responses are also integrated if they are carried out by different effector sets. On the basis of the study by Moeller and Frings (2019a), we used a prime–probe design that included two responses (A and B) to each prime and each probe. One response was always executed by the hands, and the other by the feet. If responses by the hands and feet can be integrated similarly to manual responses, we assumed that integration of hand and foot responses would occur during the prime. Then, if the first response (A) repeated at the probe, it should retrieve the second prime response (B), leading to facilitation of the second probe response (B) if the (retrieved) prime Response B was compatible to probe Response B, and to interference if the responses were incompatible. To anticipate the results, we observed evidence for binding effects between responses given via different effector sets (Exps. 1 and 2) that were smaller than the binding effects for responses carried out via the same effector set (Exp. 2).
Experiment 1
Method
Participants
The effect sizes of RR-binding effects [computed as t/sqrt(n)] were large (0.77) on average in former studies (Moeller & Frings, 2019a, b). If RR integration is not impaired by an effector switch, we therefore expected to find an effect of d = 0.77. Assuming α = .05 (one-tailed) and a power of 1–β = .85, a power analysis with the program G*Power revealed that at least 14 participants would be necessary (Faul, Erdfelder, Lang, & Buchner, 2007). Fifteen students (13 female, two male) from the University of Trier took part in the experiment (median age = 20 years, range = 18–30). One additional participant was excluded because of an extremely high error rate to the probe (16.5% for Response A and 13.4% for Response B). All participants reported normal or corrected-to-normal vision and took part in exchange for partial course credit.
Design
The design comprised two within-subjects factors, namely Response A relation (response repetition vs. response change from prime to probe) and Response B relation (response repetition vs. response change from prime to probe).
Materials
The experiment was conducted using the E-Prime 2.0 software. Instructions were shown in white on a black background on a standard TFT screen. Eight different shapes, each consisting of four overlapping lines of different lengths that could be presented in eight different colors (blue, green, red, yellow, purple, brown, and orange) were used as the stimuli. All shapes subtended a horizontal visual angle of 4.0° and a vertical visual angle of 3.7°. Two shapes were always presented simultaneously 1.2° of visual angle to the left and right of the center or the screen. Viewing distance was approximately 60 cm. Participants responded via two keys on the number pad of a computer keyboard and two pedals (Psychology Software Tools, Inc., Sharpsburg, USA). The pedals were connected to the computer via a serial response box (PST, Inc., Sharpsburg, USA), providing a 0-ms debounce period.
Procedure
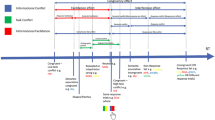
Participants were tested individually, and instructions were given on the screen. Two pedals were placed in a comfortable position on the floor in front of the participants. Participants placed their left index finger on the nine and their right index finger on the three on the number pad of a standard computer keyboard, and their feet on the pedals. They were told that they would always see two line patterns that could have identical or different shapes and identical or different colors. Their task was always to categorize first (Response A) the shapes and then (Response B) the colors of these patterns as identical or different, by successively pressing first the upper or lower key on the number pad and then the left or right pedal. For identical shapes participants pressed the lower key, and for different shapes they pressed the upper key. For identical colors, they pressed the right, and for different colors, the left foot pedal. For an example trial, see Fig. 1. The beginning of each trial was indicated by an asterisk that was presented for 500 ms in the middle of the screen. Then a plus sign appeared for 500 ms, which was followed by the prime line patterns. These were presented in white for the shape comparison and, in the case of a correct response, changed color upon Response A execution (via the index fingers). The colored shapes remained on screen until Response B (via the feet) was given. In the case of an incorrect Response A or B, a message appeared for 1,500 ms immediately following the incorrect response, reminding the participant to respond as quickly as possible but without making errors. Then a fixation mark appeared for 500 ms and was followed by the probe line patterns. The procedure in the probe was identical to that in the prime. Every 40 trials participants were allowed to take a short break, after which they resumed the task in their own time.
Sequence of events in Experiment 1 in one example trial. Participants decided for each prime and each probe whether the presented stimuli had identical or different shapes (Response A) and identical or different colors (Response B). This is an example of a Response A repetition and Response B repetition trial. Black is depicted as white and white is depicted as black; the stimuli are not drawn to scale
In Response A repetition trials (Ar), the same response was required to the shapes of the prime and probe line patterns (e.g., the prime shapes differed and the probe shapes differed). In Response A change trials (Ac), different responses were required for the categorization of the prime and probe line patterns (e.g., the prime shapes were identical and the probe shapes differed). In Response B repetition trials (Br), the same response was required to the colors of the prime and probe line patterns (e.g., the prime colors were identical and the probe colors were also identical). In Response B change trials (Bc), different responses were required to the prime and probe colors (e.g., the prime colors differed and the probe colors were identical). These relations resulted in the four conditions Response A repetition with Response B repetition (ArBr), Response A repetition with Response B change (ArBc), Response A change with Response B repetition (AcBr), and Response A change with Response B change (AcBc). Each of these conditions was presented 16 times with each of the four possible combinations of identical/different shapes and colors in the probe, resulting in 256 experimental trials. Shapes and colors were randomly assigned to the different positions/displays, with the restriction that neither shapes nor colors repeated between the prime and probe of one trial. Before the experimental block started, participants practiced their task for 16 trials (subsample of the experimental trials).
Results and discussion
For the analysis of response times (RTs), we considered only trials with correct Responses A and B to both the prime and probe. The error rate for prime responses (A or B) was 7.2%. The probe error rates were 3.9% for Response A and 3.5% for Response B (only including trials with correct previous responses). RTs more than 1.5 interquartile ranges above the third quartile of the probe Response B RT distribution of the participant (Tukey, 1977) and RTs shorter than 200 ms were excluded from the analysis. Due to these constraints, 18.9% of the trials were excluded from the RT analyses. For the mean RTs and error rates, see Table 1.
In a 2 (Response A relation: repetition vs. change) × 2 (Response B relation: repetition vs. change) multivariate analysis of variance (MANOVA) on probe Response B RTs with Pillai’s trace as the criterion, both main effects were significant: F(1, 14) = 5.45, p = .035, ηp2 = .28, for Response A relation, and F(1, 14) = 12.63, p = .003, ηp2 = .47, for Response B relation. More importantly, the interaction of Response A and Response B relation was significant as well, F(1, 14) = 8.28, p = .012, ηp2 = .37, indicating binding between the responses: Repeating Response A facilitated performance only if Response B was repeated, as well.
In the same analysis on error rates, the main effect of Response A, F(1, 14) = 7.37, p = .017, ηp2 = .35, and the interaction of Response A and Response B, F(1, 14) = 10.03, p = .007, ηp2 = .42, were significant, again indicating binding between the responses. Together, the RT and error rate data indicate that RR binding across effector sets is possible. To get an idea how these effects compare to RR bindings when responses are executed with one effector set (as in previous studies), we conducted Experiment 2.
Experiment 2
Method
Participants
Eighteen students (12 female, six male) from the University of Trier took part (median age 25.5 years; range 19–29). Two additional participants were excluded because of extremely high error rates (> 10%) to probe Response B. All participants reported normal or corrected-to-normal vision and took part in exchange for partial course credit or monetary compensation.
Design
The design comprised three within-subjects factors, namely effector set (one vs. two), Response A relation (response repetition vs. response change), and Response B relation (response repetition vs. response change).
Materials and procedure
Experiment 2 was identical to Experiment 1, with the following exceptions. Each participant worked through two blocks of 128 trials (block order was balanced across participants). In one of the blocks, the shape comparison (Response A) was carried out via the left foot (different) and the right foot (same); in the other, it was carried out via the left middle finger (different) and the right middle finger (same), which lay on keys 7 and 9 of the number pad. The color comparison (Response B) was always done via the index fingers, with the left finger lying on the upper key (5; different) and the right on the lower key (2; same) of the number pad.
Results and discussion
Only trials with correct Responses A and B to the prime and probe were entered into the RT analysis. The error rate for prime responses (A or B) was 6.6%, and the probe error rates were 2.8% for Response A and 4.0% for Response B (only including trials with correct previous responses). Due to the same constraints as in Experiment 1, 16.4% of the trials were excluded from the RT analyses. For the mean RTs and error rates, see Table 1.
In a 2 (Effector set: one vs. two) × 2 (Response A relation: repetition vs. change) × 2 (Response B relation: repetition vs. change) MANOVA on probe Response B RTs with Pillai’s trace as the criterion, the main effects for effector set, F(1, 17) = 4.53, p = .048, ηp2 = .21, and Response A relation, F(1, 17) = 12.87, p = .002, ηp2 = .43, as well as the interaction of effector set and Response A relation, F(1, 17) = 7.06, p = .017, ηp2 = .29, were significant. Importantly, the interaction of Response A and Response B relation was also significant, F(1, 17) = 20.48, p < .001, ηp2 = .55, indicating binding between the responses. This interaction was further modulated by the factor effector set, F(1, 17) = 6.37, p = .022, ηp2 = .27. Follow-up analyses revealed a larger binding effect for responses via the same effector set, F(1, 17) = 24.97, p < .001, ηp2 = .60, than for responses via different effector sets, F(1, 17) = 6.34, p = .022, ηp2 = .27; see Fig. 2. Hence, we replicated the finding that RR binding is possible even for responses executed via different effector sets. In addition, binding effects were larger for responses by one effector set.
Response–response binding effects in milliseconds, measured for probe Response B when responses were carried out by two effector sets (Exps. 1 and 2) or by one (the same) effector set (Exp. 2). Binding effects were calculated as the difference between the advantages due to Response A repetition (as compared to Response A change) for Response B change and Response B repetition trials. Error bars depict standard errors of the means
In the same analysis on error rates, only the main effect of effector set, F(1, 17) = 6.48, p = .021, ηp2 = .28, and the interaction of Response A and Response B, F(1, 17) = 7.66, p = .013, ηp2 = .31, were significant, indicating that RR binding that was not modulated by effector set switches, F < 1.
General discussion
We investigated whether responses by different effector sets can be integrated, even though responses via different effector sets are generally strongly separated (e.g., Braem et al., 2011; Moeller et al., 2015). In two experiments, we observed clear evidence that responses via different effector sets can be integrated, so that repeating one response can retrieve the other. Hence, the integration of responses is not prevented by effector-set changes within response sequences, and it is safe to assume that binding processes play a role in the coordination of sequential actions in general. We also observed that binding effects were weaker for responses executed by different effector sets than for those by the same effector set. This might point to an advantage of coordinating actions within an effector set as compared to actions with different effector sets, at least with regard to manual and pedal actions.
It has been suggested that responses are represented in terms of their action goals rather than specific motor programs (e.g., Eder et al., 2012; Prinz, 1997). Such effector-independent representation might be an explanation for why effector-set switches did not prevent RR-binding effects. Notably, the choices in our study were always “same” versus “different,” regardless of what effector set was used. With this semantic level of response representation available, the present RR-binding effects across different effector sets might have occurred at an effector-independent level, as well.
In addition, RR-binding effects for responses by different effector sets were weaker than binding effects for responses by the same effector set. This finding is in line with the assumption that responses with distinctly separate representations are less likely to be integrated than less separated responses. Multiple levels of response representation have been assumed in SR binding (Henson et al., 2014). Therefore, an additional factor might be that response representation of the integrated sequence fitted on a larger number of levels at retrieval in the effector-set repetition condition than in the effector-set change condition.
Our finding helps describe the structure of action representation in more detail. It has been suggested that event representation can be hierarchically structured. Not only are micro-events of a single response represented via bindings of their elements (i.e., SR bindings), but the same micro-events may also be integrated in larger-scale representations (see Hommel et al., 2001; Moeller & Frings, 2019a, b, for a similar view regarding the representation of task pairs: Hirsch, Nolden, & Koch, 2017). From this perspective, each Response A and B in our study can be understood as one micro-event, which were integrated with each other in a larger-scale representation for each prime. Responses A and B in the present study were not only part of different tasks (comparing shapes vs. comparing colors) but were additionally separated by being assigned to different effector sets. The latter modulation alone functions as a task switch (Philipp & Koch, 2005, 2011) and has been shown to disrupt the retrieval of SR bindings from one individual response to the other (Moeller et al., 2015). Thus, we can be quite certain that no retrieval of Response A could take place at Response B within one prime or probe. Nevertheless, hand and foot responses were integrated, indicating that larger-scale representations of action sequences can include individual (micro-)events that in turn cannot retrieve one another.
The characteristics of SR binding and learning processes appear to be similar (e.g., Giesen & Rothermund, 2014; Moeller & Frings, 2014; Singh, Moeller, & Frings, 2016). In line with this, what is known from sequence learning in sequential response time (SRT) tasks (see Abrahamse, Jiménez, Verwey, & Clegg, 2010, for a review) seems to be mirrored in the present results. In particular, sequence learning at least partly relies on the formation of response–response associations (Hoffmann, Martin, & Schilling, 2003; Nattkemper & Prinz, 1997), and moreover, this learning is effector-unspecific (Cohen, Ivry, & Keele, 1990; Willingham, Wells, Farrell, & Stemwedel, 2000). Note, however, that we found responses by different effector sets to be integrated with each other, so that the first response could retrieve the second later on. To analyze effector independence similar to that found in SRT tasks, the effector sets would need to switch between integration and retrieval of the responses. Thus, more work will be required in order to specify the relation between RR-binding and SRT-learning processes.
In conclusion, effector-set switches between sequential responses are no obstacle to integrating these responses with each other. Responses in sequences that include actions with different effectors are integrated similarly to responses in manual action sequences, even though binding effects were not as strong as those for responses by the same effector set. We suggest that binding of successive responses is a general phenomenon supporting the control of sequences of actions.
Author note
The research reported in this article was supported by grants from the Deutsche Forschungsgemeinschaft (FOR 2790 and MO 2839/2-2).
References
Abrahamse, E. L., Jiménez, L., Verwey, W. B., & Clegg, B. A. (2010). Representing serial action and perception. Psychonomic Bulletin and Review, 17, 603–623. https://doi.org/10.3758/PBR.17.5.603
Braem, S., Verguts, T., & Notebaert, W. (2011). Conflict adaptation by means of associative learning. Journal of Experimental Psychology: Human Perception and Performance, 37, 1662–1666.
Cohen, A., Ivry, R. I., & Keele, S.W. (1990). Attention and structure in sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16, 17–30. https://doi.org/10.1037/0278-7393.16.1.17
Eder, A. B., Müsseler, J., & Hommel, B. (2012). The structure of affective action representations: Temporal binding of affective response codes. Psychological Research, 76, 111–118.
Eimer, M., Schubö, A., & Schlaghecken, F. (2002). Locus of inhibition in the masked priming of response alternatives. Journal of Motor Behavior, 34, 3–10.
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. https://doi.org/10.3758/BF03193146
Frings, C., Koch, I., Rothermund, K., Dignath, D., Giesen, C., Hommel, B., … Philipp, A. (in press). Merkmalsintegration und Abruf als wichtige Prozesse der Handlungssteuerung—Eine Paradigmen-übergreifende Perspektive [Feature integration and retrieval as core processes in action control—A cross-paradigm perspective]. Psychologische Rundschau.
Giesen, C., & Rothermund, K. (2014). Distractor repetitions retrieve previous responses and previous targets: experimental dissociations of distractor–response and distractor–target bindings. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40, 645–659. https://doi.org/10.1037/a0035278
Henson, R. N., Eckstein, D., Waszak, F., Frings, C., & Horner, A. J. (2014). Stimulus–response bindings in priming. Trends in Cognitive Sciences, 18, 376–384. https://doi.org/10.1016/j.tics.2014.03.004
Hirsch, P., Nolden, S., & Koch, I. (2017). Higher-order cognitive control in dual tasks: Evidence from task-pair switching. Journal of Experimental Psychology: Human Perception and Performance, 43, 569–580.
Hoffmann, J., Martin, C., & Schilling, A. (2003). Unique transitions between stimuli and responses in SRT tasks: Evidence for the primacy of response predictions. Psychological Research, 67, 160–173.
Hommel, B., Müsseler, J., Aschersleben, G., & Prinz, W. (2001). The Theory of Event Coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences, 24, 849–878, disc. 878–937. https://doi.org/10.1017/S0140525X01000103
Logan, G. D. (1988). Toward an instance theory of automatization. Psychological Review, 95, 492–527. https://doi.org/10.1037/0033-295X.95.4.492
Moeller, B., & Frings, C. (2014). Attention meets binding: Only attended distractors are used for the retrieval of event files. Attention, Perception, & Psychophysics, 76, 959-978.
Moeller, B., & Frings, C. (2019a). From simple to complex actions: Response–response bindings as a new approach to action sequences. Journal of Experimental Psychology: General, 148, 174–183.
Moeller, B., & Frings, C. (2019b). Lost time: Bindings do not represent temporal order information. Psychonomic Bulletin and Review, 26, 325–331. https://doi.org/10.3758/s13423-018-1493-y
Moeller, B., Hommel, B., & Frings, C. (2015). From hands to feet: Abstract response representations in distractor–response bindings. Acta Psychologica, 159, 69–75.
Nattkemper, D., & Prinz, W. (1997). Stimulus and response anticipation in a serial reaction task. Psychological Research, 60, 98–112.
Philipp, A., & Koch, I. (2011). The role of response modalities in cognitive task representations. Advances in Cognitive Psychology, 7, 31–38.
Philipp, A. M., & Koch, I. (2005). Switching of response modalities. Quarterly Journal of Experimental Psychology, 58A, 1325–1338. https://doi.org/10.1080/02724980443000656
Prinz, W. (1997). Perception and action planning. European Journal of Cognitive Psychology, 9, 129–154. https://doi.org/10.1080/713752551
Rosenbaum, D. A. (1980). Human movement initiation: Specification of arm, direction, and extent. Journal of Experimental Psychology: General, 109, 444–474. https://doi.org/10.1037/0096-3445.109.4.444
Schmidt, J. R., De Houwer, J., & Rothermund, K. (2016). The parallel episodic processing (PEP) model 2.0: A single computational model of stimulus–response binding, contingency learning, power curves, and mixing costs. Cognitive psychology, 91, 82–108.
Schmidt, R. A. (1975). A schema theory of discrete motor skill learning. Psychological Review, 82, 225–260. https://doi.org/10.1037/h0076770
Singh, T., Moeller, B., & Frings, C. (2016). Five shades of grey: Generalization in distractor-based retrieval of S–R episodes. Attention, Perception, & Psychophysics, 78, 2307–2312. https://doi.org/10.3758/s13414-016-1210-8
Stoet, G., & Hommel, B. (1999). Action planning and the temporal binding of response codes. Journal of Experimental Psychology: Human Perception and Performance, 25, 1625–1640. https://doi.org/10.1037/0096-1523.25.6.1625
Tukey, J. (1977). Exploratory data analysis. Reading: Addison-Wesley.
Willingham, D. B., Wells, L. A., Farrell, J. M., & Stemwedel, M. E. (2000). Implicit motor sequence learning is represented in response locations. Memory & Cognition, 28, 366–375. https://doi.org/10.3758/BF03198552
Wright, C. E. (1990). Generalized motor programs: Reevaluating claims of effector independence. In M. Jeannerod (Ed.), Attention and performance XIII: Motor representation and control (pp. 294–320). Hillsdale: Erlbaum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Open Practice Statement
The data of the reported experiments are not openly available, and the experiments were not preregistered.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moeller, B., Frings, C. Response–response binding across effector-set switches. Psychon Bull Rev 26, 1974–1979 (2019). https://doi.org/10.3758/s13423-019-01669-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-019-01669-8