Abstract
When an unexpected event, such as a car horn honking, occurs in daily life, it often disrupts our train of thought. In the lab, this effect was recently modeled with a task in which verbal working memory (WM) was disrupted by unexpected auditory events (Wessel et al. in Nature Communications, 7, 11195, 2016). Here we tested whether this effect extends to a different type of WM—namely, visuomotor. We found that unexpected auditory events similarly decremented visuomotor WM. Moreover, this effect persisted for many more trials than had previously been shown for verbal WM, and the effect occurred for two different types of unexpected auditory events. Furthermore, we found that unexpected events decremented WM by decreasing the quantity, but not necessarily the quality, of items stored. These results showed an impact of unexpected events on visuomotor WM that was statistically robust and endured across time. They also showed that the effect was based on an increase in guessing, consistent with a neuroscience-inspired theory that unexpected events “wipe out” WM by stopping the ongoing maintenance of the trace. This new task paradigm is an excellent vehicle for further explorations of distractibility.
Similar content being viewed by others
Daily life is littered with unexpected events: the jingling of a phone’s ringtone, the honking of a car horn, the screech of a passing crow. These events are distracting, often interrupting our train of thought and jolting us away from the task at hand. A recent study investigated such distraction using a laboratory task in which working memory (WM) was interrupted by unexpected auditory events (Wessel et al., 2016). On each trial, participants encoded a letter string and were then presented with a standard tone (80%) or a novel birdsong (20%). The novel stimuli led to lower WM accuracy upon probe. Although the typical view of the underlying mechanism is that it is “caused” by an attention shift, the same authors provided a deeper insight. The novel stimulus recruited the same brain network as outright stopping of an action (Wessel & Aron, 2013; Wessel et al., 2016), and the more it did so, the greater the WM disruption (Wessel et al., 2016). It was proposed that the stop system can interrupt some forms of cognition just as it can interrupt skeletomotor activity (Wessel & Aron, 2017). This account suggests intriguing links across several disparate areas of psychology—namely attention, working memory, surprise, response inhibition, and distractibility (Banich, Mackiewicz Seghete, Depue, & Burgess, 2015; Horstmann, 2006; Leiva, Parmentier, Elchlepp, & Verbruggen, 2015; Levy & Anderson, 2002; Rissman, Gazzaley, & D’Esposito, 2009). Here we set out to more thoroughly substantiate the behavioral effect, to elaborate the paradigm with several new psychometric features, and to test several behavioral implications. (Note that, throughout, we refer to the unexpected events as “novels,” in keeping with a substantial literature: Parmentier, 2008, 2014; Parmentier, Elsley, Andres, & Barcelo, 2011).
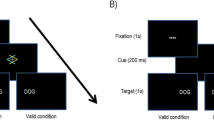
In Experiment 1 we tested whether the impact of unexpected events on cognition also applies to visuomotor WM (i.e., the coordination of visual perception and planned movement; di Pellegrino & Wise, 1993; Goodale, 1998). Accordingly, we designed a Corsi-like task (Corsi, 1972) in which participants encoded a sequence of boxes presented at different locations. They maintained this “sequence” across a delay, during which a sound occurred. This sound was mostly a standard stimulus, but occasionally it was a novel birdsong. Participants were then probed to recall the position of one of the boxes (Fig. 1).
Schematic for the main task (span test not shown). “ITI” is the intertrial interval, and “SOA” refers to the stimulus offset asynchrony. Note that for Exp. 2, the tone duration was sometimes 500 ms instead of 200 ms (10% of trials).
We established the basic effect that novels decremented visuomotor WM. We then tested (in Exp. 2) whether this effect was a generalizable feature of unexpected events. Along with the birdsong novels, we included trials in which the standard tone was simply increased in duration (cf. Näätänen, Pakarinen, Rinne, & Takegata, 2004).
In Experiment 3 we tested whether the “WM decrementing effect” was truly due to the novelty of the tone or could instead be attributable to the different sensory elements of the novels versus the standards. We now used a birdsong on every trial, with some of these remaining unexpected. We also probed how WM was impacted by novels. As above, our theory was that visuomotor WM would be maintained via cortico-basal-ganglia loops that were interrupted by unexpected-event-driven subthalamic activation. This might predict that WM itself could be partially “wiped out” (i.e., a reduction in “quantity”), which would lead to increased guessing at the probe, rather than to a change in the “quality” of the representation, which would lead to a reduction in precision. Accordingly, we used a model-fitting procedure (Suchow, Brady, Fougnie, & Alvarez, 2013) based on models of WM storage (Brady, Konkle, & Alvarez, 2011; Ma, Husain, & Bays, 2014; Zhang & Luck, 2008, 2011) to test for changes in guessing, precision, or both.
Experiment 1
Method
Participants
Sixteen students (15 female, one male; mean age 20.8 ± 2.1 years) from the University of California, San Diego (UCSD), participated for course credit. All were right-handed and had normal or corrected-to-normal visual acuity. They signed written consent according to the local ethics committee (IRB #140033).
An earlier study of the effect of unexpected events on verbal WM had shown a within-subjects novel-versus-standard effect size of Cohen’s d = 0.78 (Wessel et al., 2016). From this we estimated that 16 participants would give 90% power to detect a significant effect with alpha of .05, one-tailed.
Apparatus and stimuli
Participants sat ~22 in. in front of a 19-in. iMac personal computer (Apple) supported by a chinrest. The study was run with MATLAB 2015b (The MathWorks) and Psychophysics Toolbox 3 (Brainard, 1997). The sounds were delivered through Sennheiser HD 280 Pro headphones. Responses were recorded using an Apple mouse. The stimuli were turquoise (HEX #00EDFF) squares, measuring approximately 0.5 in. × 0.5 in. (subtended 1.3° × 1.3° of visual angle).
Procedure and design
Testing was divided into (1) determining WM span and (2) the main experiment.
The WM span procedure was designed to derive the correct span for each participant to have good sensitivity to detect WM decrements. Each trial began with a central fixation cross, around which a number of square stimuli (20 trials each of three, four, five, and six squares) were shown in succession, with each presented for 500 ms in one of 12 locations on the screen (no delay between presentation times). No locations were repeated within a trial. Then the central fixation cross was once again displayed alone for a delay of 1.4–2.9 s, jittered. This was followed by a tone, which in the case of the span test was always the standard tone—a 600-Hz sine wave presented for 200 ms—followed by a delay of 300 ms. After the delay, a number would appear in the center of screen for 500 ms, indicating which square, based on the order, the participant needed to remember. Finally, a square would reappear, in either the exact same location as the target square or a slightly different location. Participants were given 3 s to respond by indicating whether the probe square was in the same location as (“press 1”) or a different location from (“press 2”) the target square. We then calculated the number of individual squares remembered for each participant by using Cowan’s k: K = (H – FA)*N, where K is the number of items stored, H is the hit rate, FA is the false alarm rate, and N is the number of items presented (Alvarez & Cavanagh, 2004; Cowan, 2001), which was then used as set size for the main experiment.
For the main experiment, each trial proceeded as in the span test, with two key differences (Fig. 1). First, the trials were divided into two conditions: standard and novel. In the standard condition (80% of trials), the tone in the delay period was the same 600-Hz sine wave as in the span test. However, in the novel condition (20% of trials), a unique birdsong segment was presented, instead. Additionally, probe squares were no longer presented; instead the mouse cursor reappeared in the center of the screen, and participants clicked the position where they believed the target had been located. Trials were presented in a pseudorandom order, constrained so that two novel trials could never occur in a row and the first three trials of every block were standards. A total of 320 trials were presented, divided into eight blocks of 40 trials (eight of which were novel trials and 32 of which were standard trials).
Results
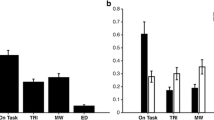
The main measure of interest was response error—the distance from the mouse click to the centroid of the target square (in degrees of visual angle). Trials with response errors more than 2.5 standard deviations from the condition mean for the block were removed as outliers. All tests were two-tailed. A two-way repeated measures analysis of variance (ANOVA) with the factors condition (standard vs. novel) and block (1 to 8) yielded a main effect of condition [F(1, 15) = 8.88, p = .009, η p 2 = .372; see Fig. 2], with response errors being significantly greater for novels than for standards. This effect persisted throughout the experiment, with no significant interaction between condition and block [F(1, 15) = 1.65, p = .130].
Response times (RTs), defined as the times between when the mouse cursor reappeared in the center of the screen and when participants clicked, did not differ between novels and standards [t(15) = 1.74, p = .103].
As an auxiliary analysis, we tested whether this WM decrement was limited to the current trial or also extended into the following trial. We calculated response errors and RTs for standard trials (t), with trials divided as a function of the previous trial (t – 1)—that is, the current trial (t) was now analyzed as either “postnovel” or “poststandard.” Response errors were significantly higher on postnovel trials (mean distance = 1.78 ± 0.44) than on poststandard trials (mean distance = 1.54 ± 0.24) [t(15) = 2.85, p = .012]. We observed no significant difference in RTs between postnovel and poststandard trials [t(15) = 0.53, p = .602].
This study thus extended the earlier verbal WM results (Wessel et al., 2016) by showing that visuomotor WM is also decremented by auditory novels. Additionally, these effects carried over into the next trial.
Experiment 2
We now tested whether the novel-induced decrement occurs for stimuli other than birdsongs.
Method
Participants
Given the partial eta-squared value of .372 in Experiment 1, 16 participants would give us 99% power to detect an effect. We thus kept N equal to 16 to be consistent across studies. Sixteen students (ten female, six male; 13 right-handed; mean age 21.0 ± 4.2 years) were recruited and gave consent as in Experiment 1.
Apparatus, stimuli, and procedure
All aspects were the same as in Experiment 1, except that (1) the set size for the experimental task was now determined as Cowan’s k + 1 (we reasoned that this would make WM even more prone to disruption), and (2) novels were subdivided into two types: Half (10% of all trials) were the 200-ms birdsong segments used in Experiment 1, and the other half (10% of all trials) were instead long tones (600-Hz sine waves) lasting 500 ms.
Results
An ANOVA was conducted with the factors condition (standard vs. novel) and block (1 to 8), with response errors and outliers being determined as in Experiment 1. Again, we found a significant main effect of condition [F(1, 15) = 24.98, p < .001, η p 2 = .625], with greater response errors for novels than for standards (see Fig. 2). There was no main effect of block [F(1, 15) = 0.77, p = .617] or block by condition interaction [F(1, 15) = 1.15, p = .337]. Furthermore, post hoc tests using the Bonferroni correction revealed that the effect was present for both types of novels (birdsong vs. standard, p = .009; long tone vs. standard, p = .001). We observed no significant effect of condition on RT [t(15) = 1.17, p = .260].
Response errors and RTs were also calculated for postnovel and poststandard trials as in Experiment 1. Response errors were significantly greater for postnovel trials (mean distance = 2.31 ± 0.59) than for poststandard trials (mean distance = 1.96 ± 0.58) [t(15) = 4.83, p < .001], although there was no significant difference in RTs [t(15) = 1.85, p = .085].
This experiment thus replicated Experiment 1, and went further by showing that a different kind of novel (a longer tone) is also capable of decrementing visuomotor WM.
Experiment 3
Method
Participants
To be consistent across all three studies, we again ran 16 participants (14 female, two male; 15 right-handed, mean age 21.8 ± 4.9 years), who were recruited and gave consent as in Experiment 1.
Apparatus, stimuli, and procedure
All aspects were the same as in Experiment 2, except that for the main task, only birdsongs were now used. These were now counterbalanced, with each acting as a standard or a novel, depending on the block (specifically, the main experiment consisted of nine blocks [360 trials] with nine unique birdsong segments used throughout the experiment; for each block, one of the birdsong segments served as the standard, with the other eight serving as novels, and the standard rotated across blocks). We also increased the number of trials (with now 72 instead of 64 novels) and standardized the stimulus locations to a fixed eccentricity (i.e., each box occurred at some point on an invisible circle ringing the screen).
Data analysis
The increased number of trials and standardized stimulus locations now allowed us to attempt to measure differences in capacity and precision. For each trial, we calculated the response error as the angular distance between the correct and reported locations. We then modeled these error distributions as a mixture of a circular normal distribution (centered around the correct location) and a uniform distribution (random guesses), with two parameters—a mixture parameter (G, indicating how many trials were “guesses”Footnote 1) and an SD parameter (indicating how wide the normal distribution was—i.e., precision; Zhang & Luck, 2008). The data were fit using MemToolbox (Suchow et al., 2013), and parameter estimates were derived using Markov chain Monte Carlo simluations to find the maximum a posteriori values. The model was fit separately for each participant and task condition (novel vs. standard). We also analyzed the data using a modified model that estimated how frequently participants reported one of the other square locations as the target location (“swap errors”; Bays et al., 2009). The results converged across the two models, and parameter estimates for this alternative model are listed in the supplementary materials.
Results
An ANOVA with the factors condition (standard vs. novel) and block (1 to 9) revealed a significant effect of condition [F(1, 15) = 17.19, p = .001, η p 2 = .534]. This showed, again, that response errors increased on novel relative to standard trials (see Fig. 2). We found no significant main effect of block [F(1, 15) = 0.84, p = .566] or interaction [F(1, 15) = 1.84, p = .076]. Interestingly, the interaction was at trend level, and this trend was in the direction of the unexpected-event-induced decrement in WM increasing over time. Indeed, the effect was significant even in the last block [t(15) = 2.79, p = .014]. Again, no significant effect of condition emerged in RTs [t(15) = 1.57, p = .137]. There was also no significant difference between postnovel and poststandard trials with regard to either response errors [t(15) = 1.51, p = .151] or RTs [t(15) = –1.15, p = .269].
Fitting the mixture model to the response error data revealed that participants guessed significantly more often for novels (16.1%) than for standards (12.6%) [t(15) = 2.44, p = .028; Fig. 3B, left]. There was no significant difference in the quality with which the locations were stored, with SD estimates of 10.0° for novels and 9.7° for standards [t(15) = 0.53, p = .606; Fig. 3B, right]. We note, however, that such a model can have more power to detect differences in guessing than in precision (Fougnie, Suchow, & Alvarez, 2012; Suchow, Fougnie, Brady, & Alvarez, 2014), and there were only 72 novels. Yet Monte Carlo simulations showed that with this number of participants and this number of trials per condition, we had 91% power to detect an SD change as small as 1°.
(A) Response error histograms for the novel condition (left) and the standard condition (right), collapsed over all participants. The solid lines indicate the standard mixture model. (B) Model parameter estimates for each condition for the guess rate (left) and standard deviation (also known as the precision; right). Error bars represent within-subjects standard deviations.
General discussion
An earlier study showed that novel sounds decrement verbal WM and suggested that this was caused by a neural mechanism (Wessel et al., 2016). Here we expanded on that work by testing a visuomotor WM paradigm with several more sophisticated features of the experimental design. In Experiment 1 we showed that visuomotor WM is also decremented by auditory novels (birdsongs). In Experiment 2 we again showed that auditory novels (birdsongs) decrement WM, as do standard tones that are merely extended in time. In Experiment 3 we showed that the effect of the unexpected events, and not novel-versus-standard physical stimulus differences, was what counted (Table 1). The effect sizes for the novel-decrementing WM results were in all cases greater than .26, which is typically defined as large (partial eta-squareds of .372 [Exp. 1], .625 [Exp. 2], and .534 [Exp. 3]). We also showed that novels decrement WM by increasing guessing. The fact that RTs to the probe were not generally increased following novels versus standards does not contradict the idea that novels recruit a stopping system—other research has suggested that the stopping system is recruited very quickly and briefly after a novel stimulus (Wessel & Aron, 2017). Moreover, the decrement did not abate with time—indeed, in Experiment 3 the decrement even increased across time. To further test this, we calculated the split-half reliability for WM accuracy (i.e., response error) in each of the three experiments. Although reliability was low for Experiment 1 (Spearman–Brown split-half reliability coefficient [r SB] = .29), it was quite high for both Experiments 2 (r SB = .66) and 3 (r SB = .71), providing further evidence that these effects are statistically stable across trials. Taken together, these results show statistically robust, and enduring, impacts of novels on visuomotor WM. They also show an increase in guessing, which is consistent with a neuroscience-inspired theory of the mechanism of distraction.
As an auxiliary analysis, we examined response errors on standard trials following novel trials, as compared to standard trials following other standard trials. Response errors increased in the former trial type in two of the three experiments (Exps. 1 and 2). This makes sense, even within the context of a brief, transient stop process being recruited by the unexpected event, as evidence of a later potential post-error slowing or distraction effect. Conceptually, this is consistent with the idea that the unexpected event during the delay period on trial n recruits the stop network and impacts the maintenance of WM, thus causing the WM decrement on that trial. Then, general distraction and loss of attention may be a secondary consequence of the unexpected event and may impact encoding on trial n + 1. Because this “trial-after” effect was only present in two of the three experiments and was not the main focus of the study, we do not discuss it further.
The large, enduring effect of novel-induced distraction seen in this study is to be contrasted with the effects for the verbal WM paradigm (Wessel et al., 2016). There, although significant effects did emerge in several participant groups, the effects were not of so dramatic a magnitude and did not last aso long. We conjecture that visuomotor WM is more fragile—that is, rehearsing a motor sequence of spatial locations is more easily interruptible than verbal WM, which is presumably rehearsed via the phonological loop. We also showed that even stimuli that are well-balanced in their physical characteristics interrupt WM when they are unexpected. All of these features make the present paradigm an excellent vehicle for further explorations of distractibility—it produces a reliable effect that can be studied across many trials and is not confounded by stimulus differences.
It is notable that novels decremented WM by increasing guessing, without any apparent effect on precision. This is interesting in relation to our neural theory that visuomotor WM is maintained via thalamocortical drive. A stop-induced interruption in that activation might be expected to produce a disruption of memory for a visuomotor sequence, which may manifest as a loss of one of more item representations rather than as an overall “blurring” of precision.
An interesting question is whether there is something special, with regard to interruptibility, about visuomotor or verbal WM relative to other kinds of WM, such as for colors or faces. It is likely that both visuomotor and verbal WM are motor-based. In that sense, an unexpected-event-driven stop system might interrupt them in the same way that it “suppresses” the skeletomotor system (Wessel & Aron, 2013). More generally, this theory provides new perspectives on the connection between the basal ganglia and WM (cf. Chatham & Badre, 2015).
Notably, the impact of distraction on WM has been difficult to demonstrate empirically, and many of those studies that do show an effect use a distractor (e.g., visual) which 'shapes' the (visual) WM representation (Ester, Zilber, & Serences, 2015; Hakun & Ravizza, 2016; Rademaker, Bloem, De Weerd, & Sack, 2015; Yoon, Curtis, & D’Esposito, 2006; however, see Awh, Vogel, & Oh, 2006; Cools, Miyakawa, Sheridan, & D’Esposito, 2010; Ester et al., 2015; Gazzaley, Cooney, Rissman, & D’Esposito, 2005; McNab & Klingberg, 2008; Zanto & Gazzaley, 2009). In the real world, however, distraction is often in a different modality than the target information (e.g., a cell phone chirping while you are trying to remember the exit from the highway), and moreover, the distractor is typically unexpected. In these ways, the present approach is a more realistic paradigm for understanding distraction, and as we have shown, the distraction it produces is statistically robust and endures across trials. Further studies could test whether unexpected events interrupt visuomotor WM and induce guessing via “erasing” current traces through the stopping system, whether this extends to nonmotor forms of WM, and whether this explains clinical conditions of under- and over-distractibility.
Change history
17 July 2018
The authors are retracting this article (Finzi et al., 2018) because after publication they discovered a mistake in the behavioral analysis.
References
Alvarez, G. A., & Cavanagh, P. (2004). The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science, 15, 106–111. doi:10.1111/j.0963-7214.2004.01502006.x
Awh, E., Vogel, E. K., & Oh, S. H. (2006). Interactions between attention and working memory. Neuroscience, 139, 201–208. doi:10.1016/j.neuroscience.2005.08.023
Banich, M. T., Mackiewicz Seghete, K. L., Depue, B. E., & Burgess, G. C. (2015). Multiple modes of clearing one’s mind of current thoughts: Overlapping and distinct neural systems. Neuropsychologia, 69, 105–117. doi:10.1016/j.neuropsychologia.2015.01.039
Bays, P. M., Catalao, R. F., & Husain, M. (2009). The precision of visual working memory is set by allocation of a shared resource. Journal of Vision, 9(10), 7.1–11. doi:10.1167/9.10.7
Brady, T. F., Konkle, T., & Alvarez, G. A. (2011). A review of visual memory capacity: Beyond individual items and toward structured representations. Journal of Vision, 11(5), 4. doi:10.1167/11.5.4
Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. doi:10.1163/156856897X00357
Chatham, C. H., & Badre, D. (2015). Multiple gates on working memory. Current Opinion in Behavioral Science, 1, 23–31. doi:10.1016/j.cobeha.2014.08.001
Cools, R., Miyakawa, A., Sheridan, M., & D’Esposito, M. (2010). Enhanced frontal function in Parkinson’s disease. Brain, 133, 225–233. doi:10.1093/brain/awp301
Corsi, P. M. (1972). Human memory and the medial temporal region of the brain. Dissertation Abstracts International: Section B. Sciences and Engineering, 34(2), 891.
Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences, 24, 87–114. doi:10.1017/S0140525X01003922. disc. 114–185.
di Pellegrino, G., & Wise, S. P. (1993). Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. Journal of Neuroscience, 13, 1227–1243.
Ester, E. F., Zilber, E., & Serences, J. T. (2015). Substitution and pooling in visual crowding induced by similar and dissimilar distractors. Journal of Vision, 15(1), 4. doi:10.1167/15.1.4. 1–14.
Fougnie, D., Suchow, J. W., & Alvarez, G. A. (2012). Variability in the quality of visual working memory. Nature Communications, 3, 1229. doi:10.1038/ncomms2237
Gazzaley, A., Cooney, J. W., Rissman, J., & D’Esposito, M. (2005). Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience, 8, 1298–1300. doi:10.1038/nn1543
Goodale, M. A. (1998). Visuomotor control: Where does vision end and action begin? Current Biology, 8, R489–R491.
Hakun, J. G., & Ravizza, S. M. (2016). Ventral fronto-parietal contributions to the disruption of visual working memory storage. NeuroImage, 124, 783–793. doi:10.1016/j.neuroimage.2015.09.056
Horstmann, G. (2006). Latency and duration of the action interruption in surprise. Cognition and Emotion, 20, 242–273. doi:10.1080/02699930500262878
Leiva, A., Parmentier, F. B., Elchlepp, H., & Verbruggen, F. (2015). Reorienting the mind: The impact of novel sounds on go/no-go performance. Journal of Experimental Psychology: Human Perception and Performance, 41, 1197–1202. doi:10.1037/xhp0000111
Levy, B. J., & Anderson, M. C. (2002). Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences, 6, 299–305.
Ma, W. J., Husain, M., & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience, 17, 347–356. doi:10.1038/nn.3655
McNab, F., & Klingberg, T. (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103–107. doi:10.1038/nn2024
Näätänen, R., Pakarinen, S., Rinne, T., & Takegata, R. (2004). The mismatch negativity (MMN): Towards the optimal paradigm. Clinical Neurophysiology, 115, 140–144. doi:10.1016/j.clinph.2003.04.001
Parmentier, F. B. (2008). Towards a cognitive model of distraction by auditory novelty: The role of involuntary attention capture and semantic processing. Cognition, 109, 345–362. doi:10.1016/j.cognition.2008.09.005
Parmentier, F. B. (2014). The cognitive determinants of behavioral distraction by deviant auditory stimuli: A review. Psychological Research, 78, 321–338. doi:10.1007/s00426-013-0534-4
Parmentier, F. B., Elsley, J. V., Andres, P., & Barcelo, F. (2011). Why are auditory novels distracting? Contrasting the roles of novelty, violation of expectation and stimulus change. Cognition, 119, 374–380. doi:10.1016/j.cognition.2011.02.001
Rademaker, R. L., Bloem, I. M., De Weerd, P., & Sack, A. T. (2015). The impact of interference on short-term memory for visual orientation. Journal of Experimental Psychology: Human Perception and Performance, 41, 1650–1665. doi:10.1037/xhp0000110
Rissman, J., Gazzaley, A., & D’Esposito, M. (2009). The effect of non-visual working memory load on top-down modulation of visual processing. Neuropsychologia, 47, 1637–1646. doi:10.1016/j.neuropsychologia.2009.01.036
Suchow, J. W., Brady, T. F., Fougnie, D., & Alvarez, G. A. (2013). Modeling visual working memory with the MemToolbox. Journal of Vision, 13(10), 9. doi:10.1167/13.10.9
Suchow, J. W., Fougnie, D., Brady, T. F., & Alvarez, G. A. (2014). Terms of the debate on the format and structure of visual memory. Attention, Perception, & Psychophysics, 76, 2071–2079. doi:10.3758/s13414-014-0690-7
van den Berg, R., Shin, H., Chou, W. C., George, R., & Ma, W. J. (2012). Variability in encoding precision accounts for visual short-term memory limitations. Proceedings of the National Academy of Sciences, 109, 8780–8785. doi:10.1073/pnas.1117465109
Wessel, J. R., & Aron, A. R. (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33, 18481–18491. doi:10.1523/JNEUROSCI.3456-13.2013
Wessel, J. R., & Aron, A. R. (2017). On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron, 93, 259–280. doi:10.1016/j.neuron.2016.12.013
Wessel, J. R., Jenkinson, N., Brittain, J.-S., Voets, S. H. E. M., Aziz, T. Z., & Aron, A. R. (2016). Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nature Communications, 7, 11195. doi:10.1038/ncomms11195
Yoon, J. H., Curtis, C. E., & D’Esposito, M. (2006). Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage, 29, 1117–1126. doi:10.1016/j.neuroimage.2005.08.024
Zanto, T. P., & Gazzaley, A. (2009). Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience, 29, 3059–3066. doi:10.1523/JNEUROSCI.4621-08.2009
Zhang, W., & Luck, S. J. (2008). Discrete fixed-resolution representations in visual working memory. Nature, 453, 233–235. doi:10.1038/nature06860
Zhang, W., & Luck, S. J. (2011). The number and quality of representations in working memory. Psychological Science, 22, 1434–1441. doi:10.1177/0956797611417006
Author note
The authors thank Fabrice Parmentier and Sirawaj Itthiipuripat for helpful advice on the task design, and Francesco Marini for programming. This research was funded by grants from the National Institutes of Health (R21 NS085543) and the James S. McDonnell Foundation (#220020375).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are retracting this article because after publication they discovered a mistake in the behavioral analysis. This affects the results shown in Figure 2 and Table 1. The mistake is that, at the single subject level, the mean response error variable was calculated for novels and standards after removing outliers separately for these two conditions. However, because there were different numbers of novel and standard trials, this method inflates the mean difference between conditions. Specifically, it produces a bias towards an increased response error on novels vs. standards, as seen in Figure 2, which we confirmed with simulations. When outliers were removed in an appropriate fashion the difference between means across all three experiments was much reduced and not significant. However, single-subject analysis clearly shows that novel trials have an increased proportion of trials with a large response error (i.e. there is a mixture of two distributions) compared to standards. The mixture modeling approach of Experiment 3 captures this, and the increased guessing rate for novels vs. standards remains significant as reported in the paper. Unfortunately, the authors cannot fit the mixture model to the data from Experiments 1 and 2 as the position of stimuli on the screen was not set up to be equidistance (as they were for Experiment 3). In summary, the authors have confidence in a core result from the paper: that novel events increasing guessing when they are presented in the delay period of a visuomotor task. However, the effect is more subtle that they thought, and as Figure 1 and Table 2 have incorrect values they are retracting this article. The authors sincerely regret any inconvenience to readers. All authors agree with this retraction.
About this article
Cite this article
Finzi, R.D., Postle, B.R., Brady, T.F. et al. RETRACTED ARTICLE: Unexpected events disrupt visuomotor working memory and increase guessing. Psychon Bull Rev 25, 651–657 (2018). https://doi.org/10.3758/s13423-017-1319-3
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-017-1319-3