Abstract
This report is part of a larger project examining associative interference as a function of the nature of the interfering and target associations. Lick suppression experiments with rats assessed the effects of context shifts on proactive outcome interference by latent inhibition (LI) and Pavlovian conditioned inhibition (CI) treatments on subsequently trained Pavlovian conditioned excitation treatment. LI and CI were trained in Context A during Phase 1, and then excitation treatment was administered in Context B during Phase 2, followed by tests for conditioned excitation in Contexts A, B, or C. Experiment 1 preliminarily established our LI and CI treatments and resulted in equally retarded acquisition of behavioral control when the target cue was subsequently trained as a conditioned excitor and tested in Context A. However, only CI treatment caused the target to pass a summation test for inhibition. Centrally, Experiment 2 consisted of LI and CI treatments in Context A followed by excitatory training in Context B. Testing found low excitatory control by both LI and CI cues in Context A relative to strong excitatory control in Context B, but CI treatment transferred to Context C more strongly than LI treatment. Experiment 3 determined that LI treatment failed to transfer to Context C even when the number of LI trials was greatly increased. Thus, first-learned LI appears to be relatively context specific, whereas first-learned CI generalizes to a neutral context. These observations add to existing evidence that LI and CI treatments result in different types of learning that diverge sharply in transfer to a novel test context.
Similar content being viewed by others
There is a wide variety of two-phase associative interference designs that historically have been treated independently of one another. Dimensions on which interference can vary include outcome versus cue interference (i.e., one cue paired with two outcomes vs. two cues paired with one outcome) and retroactive interference versus proactive interference, as well as the nature or quality of the interfering information (see Polack, Jozefowiez, & Miller, 2017). Here, we focus on this last factor and compare the degree of proactive outcome interference produced by different types of nonreinforced experience with the target cue during Phase 1. Specifically, reinforcement of the target cue in Phase 2 was preceded by nonreinforcement of the target cue in Phase 1 in the form of either conditioned inhibition (CI) training (i.e., the target conditioned stimulus [CS] paired with the omission of an expected specific outcome) or latent inhibition (LI) treatment (i.e., nonreinforced presentation of the target CS in the absence of the expectation of any specific outcome).
The present article is a small part of our long-term goal of assessing whether different types of two-phase associative interference designs are subject to common or different rules. Specifically, we directly compared context specificity of LI treatment and CI training in Phase 1 when excitatory conditioning was administered in Phase 2. Because we assessed the consequences of the excitatory conditioning that occurred in Phase 2, the present series is concerned with proactive outcome interference by CI and LI treatment that occurred during Phase 1 with conditioned excitatory responding. In this framework, LI treatment prior to excitatory conditioning and CI training prior to excitatory conditioning each constitute instances of outcome interference in which the interfering outcome consists of the absence of an unconditioned stimulus (US).
An LI procedure consists of nonreinforcement (i.e., noUS, aka Outcome 1) of the target cue during Phase 1 (i.e., CS preexposure) and reinforcement of the target cue with the US (i.e., Outcome 2) during Phase 2 (Lubow & Moore, 1959). This arrangement is well known to produce a decrement in excitatory responding to the cue relative to novel cues that were not preexposed during Phase 1.
A retardation test for CI (Rescorla, 1969) following CI training constitutes another instance of associative proactive outcome interference between the presence and absence of an outcome. During Pavlovian CI training (Pavlov, 1927), interspersed reinforced and nonreinforced trials occur, which can be represented as A–US / AX–NoUS, where A is the training excitor, X is the target cue, and the absence of the US on the AX–NoUS trials is Outcome 1. To assess retarded acquisition of behavioral control to the putative conditioned inhibitor (X), X is subsequently paired with the US (X–US). These X–US pairings constitute cue Outcome 2 training, with the US serving as Outcome 2, which is in conflict with the expectation of noUS (i.e., Outcome 1) experienced during inhibitory training. A decrement in excitatory responding, relative to a cue lacking the initial CI training, supports the claim that CI had been learned. However, a summation test with a transfer conditioned excitor is needed to rule out a decrease in attention to the CI (e.g., Hearst, 1972; Rescorla, 1969).
Although both an LI cue and a CI cue exhibit retarded emergence of excitatory behavioral control, they differ in that an LI cue (relative to a neutral stimulus) does not suppress responding to a transfer conditioned excitor during a negative summation test (Reiss & Wagner, 1972; Rescorla, 1971), whereas a CI cue does reduce responding to a transfer excitor (Rescorla, 1969). This observation suggests that a different associative structure underlies learning that occurs during LI treatment than that which underlies learning during CI training (Lorden, Rickert, & Berry, 1983; Reiss & Wagner, 1972; Rescorla, 1971).
A central goal in the study of outcome interference is to understand the factors that determine which of the conflicting associations will be expressed at test. Toward this goal, many studies have examined the role of the context as a determinant of which memory will be expressed. Most of these studies have focused on situations in which the cue is first reinforced in Context A and subsequently nonreinforced in Context B (i.e., extinction; e.g., Bouton & Bolles, 1979). There is considerable research on renewal, which shows that a context shift between extinction treatment and testing often enhances expression of initial training. Moreover, administration of Phase 1 excitatory training and the Phase 2 extinction treatment in different contexts produces more recovery of the Phase 1 association (i.e., less retroactive interference) at test (ABA and ABC renewal) than when Phase 1 and Phase 2 treatment occur in the same context (AAC renewal; e.g., Polack, Laborda, & Miller, 2013).
Similar context specificity for LI (in which the order of nonreinforcement and reinforcement is reversed from that of extinction) has been reported by Channell and Hall (1983); Hall and Channell (1985); Lovibond, Preston, and Mackintosh (1984); and Swartzentruber and Bouton (1986), all of whom observed an attenuated LI effect when LI treatment and excitatory conditioning occurred in different contexts. Collectively, these studies indicate that when nonreinforcement and excitatory conditioning occur in different contexts, responding to the target cue in each of these contexts largely reflects the subjects’ prior experience in the test context. That is, the test context is seemingly used to help resolve the ambiguity experienced across the two phases of training.
Given that our central goal was to compare the context specificity of proactive interference by nonreinforcement of the target cue (LI and CI) on excitatory conditioning when testing occurred in a neutral context, we wanted a situation in which there would be a high degree of context specificity when testing occurred in either of the treatment contexts. Based on evidence from investigations of extinction, we assumed that our administrating the nonreinforcement and excitatory training in different contexts would yield more context specificity than if both types of training occurred in a single context. This extrapolation from the context specificity of extinction to that of LI finds support in reports by Channell and Hall (1983), Hall and Channell (1985), Lovibond et al. (1984), and Swartzentruber and Bouton (1986), all of whom found that the LI effect was attenuated when LI treatment and excitatory conditioning occurred in different contexts.
In hindsight, it is not surprising that when subjects are exposed to inconsistent information across two distinctive contexts (e.g., CS–NoUS in one context during Phase 1 and CS–US in the other context during Phase 2), they learn to use the two contexts as discriminative stimuli and respond accordingly when tested on the CS in the context of either Phase 1 or Phase 2 training. When testing occurs in a third and neutral context, whether one observes proactive interference or retroactive interference is less obviously predictable. The weak responding that is observed when testing occurs in the context of LI training, following a context shift between LI and excitatory training, suggests that both associations are simultaneously retained. Alternatively, it is possible that the decrement in responding in the LI context was due to generalization decrement. However, the strong responding to cue LI in Context C argues against generalization decrement accounting for the difference in responding to an LI cue between Contexts B and A. Assuming two independent memories, one of LI treatment and the other of reinforcement, testing in a neutral context would seem to be a pure test of conflict between the two opposing phases of training; however, there are some potential complicating factors. For example, there may be a response bias favoring the first-learned relationship concerning the CS (i.e., LI). Alternatively, the CS–US association, having involved reinforcement, may have an inherent advantage; additionally, recency might favor expression of the CS–US association.
Given nonreinforcement of a CS in one context followed by excitatory conditioning of the CS in a second context, Swartzentruber and Bouton (1992) observed less responding to the CS in a third (neutral) context relative to the excitatory conditioning context; yet some degree of responding to the CS was observed in the neutral context. Thus, the LI effect at least partially transferred to the neutral context (or alternatively stated, the excitatory conditioning of Phase 2 only partially transferred to the neutral context). But Swartzentruber and Bouton did not test the CS back in the context of LI treatment, so one cannot determine the degree to which LI transferred to the neutral context. Without a comparison to the amount of LI in the context where presumably it would have been maximal, we cannot be sure how much, if any, of the LI effect failed to transfer to the neutral context.
In a subsequent, notably well controlled series of experiments, Westbrook, Jones, Bailey, and Harris (2000, Experiment 5) comprehensively assessed the contextual control of LI and found that testing a CS in a context associatively neutral with respect to that particular CS yielded responding roughly midway between the low responding to the CS observed in the context of LI and the high responding to the CS seen in the context of excitatory training. That is, Westbrook et al. observed an ABC context shift effect for LI that resulted in less responding at test relative to an ABB control condition (i.e., the low responding observed during testing in the neutral context was more consistent with prior LI training in Context A than was responding at test in Context B, the context of excitatory training). Similarly, Miller, Laborda, Polack, and Miguez (2015, Experiment 2) tested a latently inhibited target cue in both the context of latent inhibition treatment and a context associatively neutral with respect to the target CS. They observed weak responding in the context of latent inhibition but generalization of excitation in the associatively neutral context. Thus, the report of Miller et al. also suggests that learning about nonreinforcement, even when it is the first-learned relationship concerning the CS (i.e., in the form of LI training) is at least partially context specific.
Relative to studies of the context specificity of the LI effect, the context specificity of CI training experienced prior to excitatory conditioning has been far less explored. Nelson (2002) observed that rats that received CI training with a target CS, followed by excitatory conditioning of that CS in the same context, exhibited less excitatory responding to the CS when it was presented outside of that training context. Here, the weak responding to the CS outside the context of inhibitory training constituted at least partial AAB renewal of CI relative to an AAA control condition (or alternatively stated, weak transfer of excitatory training to the neutral context). Thus, first-learned CI appears to partially transfer to a neutral context. Seeking additional research in which CI training in one context was followed by excitatory conditioning in a second context, the closest we could identify was a paper by Sissons and Miller (2009). They manipulated the similarity of the test context to the CI and excitatory conditioning contexts by varying the retention interval that preceded testing (i.e., they manipulated the temporal context). If one views the passage of time as creating a new temporal context (Bouton, 1993), then a delayed test is effectively an assessment of ABC renewal of CI, whereas an immediate test serves as an ABB control condition. Sissons and Miller observed that CI transferred strongly to a different temporal context (i.e., they saw what appeared to be strong recovery of CI training following a temporal context shift, i.e., delayed testing).
Although there is already literature concerning the context specificity of LI (e.g., Hall and Channell, 1985; Lovibond et al., 1984; Swartzentruber & Bouton, 1986; Westbrook et al., 2000) and CI (e.g., Nelson, 2002; Sissons & Miller, 2009), these prior studies investigated LI and CI individually and used different parameters, which invariably leads to differences in the strength of LI and CI, thereby confounding comparisons of differences in context specificity between LI and CI. Here, we examined them within the same experiment and took steps to produce initially comparable amounts of LI and CI training. Our central question was the degrees of context specificity of LI and of CI training administered in one context during Phase 1 when excitatory training occurs in a different context in Phase 2. Hence, in the present series, LI of one target cue and CI treatment of a second target cue occurred in one context (Context A) during Phase 1, followed by excitatory training of both target cues in a second context (Context B) during Phase 2. We then tested for excitatory control in Context A, Context B, and an associatively neutral Context C.
Experiment 1
Given that the goal of this series was to examine the context specificity of associative interference by prior LI or CI training on subsequent excitatory training, we first needed to identify comparable baseline LI and CI effects, as assessed by retardation of excitatory responding. Experiment 1 was a preliminary study designed to assess similarity in retardation produced by LI and CI treatments (see Table 1). Following LI and CI treatments in Context A during Phase 1, we gave excitatory training in Context B during Phase 2 to parallel what we later did in Experiment 2. Testing occurred in Context A to avoid a ceiling effect for stimulus control that was apt to occur in Context B. Our singular goal in Experiment 1 was to obtain similar levels of stimulus control by LI and CI on the retardation-of-acquisition test so we might establish parameters for equal response levels across those conditions for the subsequent assessment of transfer of LI and CI to other contexts (i.e., Context B and neutral Context C) in Experiment 2. Moreover, there was a test for negative summation on both target CSs to establish that the CI training procedure had, in fact, made the CI cue into an effective conditioned inhibitor. In addition to cues LI and CI, we included a neutral stimulus (NS) that provided a measure of acquisition on the retardation test in the absence of any retardation arising from the LI or CI treatments. Furthermore, a few prior exposures to the LI, CI, and NS cues were given at the beginning of the experiment to minimize external inhibition on the summation test.
Method
Subjects
The subjects were 36 male and 36 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 202–298 g for males and 178–234 g for females. Subjects were randomly assigned to one of six subgroups (ns = 12), counterbalanced within subgroups for sex. The animals were individually housed in standard hanging stainless-steel wire-mesh cages in a vivarium maintained on a 16/8-hr light/dark cycle. Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 30 min per day following a progressive deprivation schedule initiated 4 days prior to the start of the study. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Thirty-six chambers of three distinct types were used in this experiment. Each subject only experienced two of the contexts, but the identities of these two experimental contexts were counterbalanced (using a Latin square) across the three different types of chambers for consistency with the subsequent experiments. Chamber R was rectangular, measuring 24.0 cm × 9.0 cm × 12.5 cm (l × w × h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor was composed of stainless steel rods measuring 0.5 cm in diameter, spaced 1.3 cm apart, center to center. The rods were connected by NE-2 bulbs that allowed for the delivery a 0.8-mA, 0.5-s constant current footshock produced by a high voltage AC circuit in series with a 1.0-MΩ resistor.
Chamber V was 27-cm long, 29.5-cm high, 21.5-cm wide at the top, and 5.5-cm wide at the bottom. The floor was composed of two 27-cm long plates, 2 cm wide, with a 1.5 cm gap between the two plates. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the sidewalls were stainless steel. A 0.8-mA, 0.5-s constant-current footshock could be delivered through the metal walls and floor of the chamber.
Chamber O measured 30 cm × 25 cm × 32 cm (l × w × h). Two of the walls of each chamber were made of Plexiglas and two of stainless steel. The floor was constructed of 0.5 cm in diameter rods, spaced 2 cm center to center, and connected by NE-2 neon bulbs that allowed a 0.5-s, 0.8-mA, constant-current footshock to be delivered.
Each of the 36 chambers was housed in a light-attenuating and sound-attenuating environmental chest that was dark except when a light CS was presented. Three 45-Ω speakers on the inside and back walls of the environmental chests could deliver a click train (6 Hz), a compound tone (composed of 500 Hz and 520 Hz), and white noise. Additionally, a SonAlert mounted on the ceiling of each environmental chest was able to deliver a high-frequency (1900 Hz) tone. In all chambers, the clicks, compound tone, white noise, and SonAlert were delivered at 6 dB above background as measured on the C scale. Ventilation fans in each enclosure provided a constant background noise (76 dB on the C scale). A visual stimulus that consisted of a flashing light (0.25 s on / 0.25 s off) could also be presented. The light was provided by a 25-W bulb (Chamber R), a 100-W bulb (Chamber V), or a 75-W bulb (Chamber O), nominal at 120 VAC but driven at 80 VAC. The bulbs were mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. Given the differences in opaqueness of the chamber walls, these three lights were of approximately equal brightness inside the different types of chambers.
All O, R, and V chambers could be equipped with a water-filled lick tube that extended 1 cm into a cylindrical niche, which was 4.5 cm in diameter, left–right centered, with its bottom 1.75 cm above the floor of the apparatus and 5.0 cm deep. In all chambers there was a photobeam detector 1 cm in front of the lick tube that was broken whenever the subject licked the tube. The clicks, tone, and SonAlert served as the conditioned inhibitor (CI), the latent inhibitor (LI), and novel stimulus (NS), counterbalanced using a Latin square within each subgroup and followed the additional rule that on Test 1 (i.e., the summation test), the test stimulus was always the white noise that served as Transfer excitor (T) plus the clicks. On Test 2 (i.e., the retardation test), the stimulus tested in each subgroup was the clicks or SonAlert with equal frequency within a subgroup. Thus, on Test 1, all subjects received identical experience, and no stimulus was tested more than once. The flashing light served as the training excitor (L) used during Pavlovian CI training. All stimuli were 20 s in duration throughout training. The footshock served as the US.
Procedure
Acclimation
On Day 1, all subjects received exposure to Context A and Context B, during two 60-min sessions, one context per session. The order in which subjects were acclimated to the contexts (Context A first vs. Context B first) was counterbalanced within each of the six subgroups. There were 2 hours between these acclimation sessions. Subjects were given free access to water from lick tubes in each context on these days to establish a stable baseline level of drinking behavior, which would serve as the dependent variable during testing. In Context A, each subject additionally received one presentation of each stimulus—T, NS, L, CI, and LI—in the following order: flashing light, clicks, tone, white noise, and SonAlert, with onset at 10, 20, 30, 40, and 50 min into session, respectively. The aim of these exposures was to reduce neophobia of the cues and facilitate discrimination of elements that were going to be presented in compound during the experiment.
Phase 1 (acquisition)
On Days 2–6, all subjects received training in Context A. On Days 2–5, sessions were 120 min in duration. On Days 2–5, all subjects received 2 consecutive days of either Phase 1A followed by 2 days of Phase 1B training, or vice versa, with order of treatment counterbalanced within each subgroup, but sex and type of chamber was partially confounded with order of treatment. In Phase 1A, subjects received Pavlovian CI training with cue CI in Context A, which consisted of 10 reinforced presentations of L and 50 nonreinforced presentations of the simultaneous compound of cues CI and L per day (mean intertrial interval [ITI] = 100 s). In Phase 1B, subjects received LI training in Context A, which consisted of 60 daily nonreinforced presentations of cue LI (mean ITI = 100 s). Additionally, on Days 2–5, all subjects also received 120-min daily exposure to Context B, during which no stimuli were scheduled to appear. The session in Context B began about 120 min following the end of each subject’s Context A session. On Day 6 (Phase 1C), all subjects received transfer excitor training in Context A, which consisted of four reinforced presentations of T at 12, 24, 36, and 48 min into the 60-min session. Additionally, on Day 6, all subjects received 60 min of exposure to Context B during which no stimuli were scheduled to occur. Context B exposure occurred about 120 min after the end of training in Context A and was intended to equate exposure to Contexts A and B during Phase 1. The equating of context exposures was also done in each subsequent phase of training in the experiment. Equating exposure was necessary in subsequent experiments and was done here only to maximize similarity across experiments.
Reacclimation
On Days 7–9 and 12–13, all subjects received exposure to Contexts A and B to restore baseline drinking behavior (with order of context exposure counterbalanced with respect to subgroup), which might have been disrupted by the footshocks administered in Context A. The reacclimation procedure was also intended to reduce any differences between Contexts A and B in associative strength to the US. This treatment consisted of daily 60-min sessions in which subjects received free access to water and no nominal stimulus was scheduled to appear.
Test 1 (summation test)
On Day 10, all subjects were tested for conditioned lick suppression to a test compound in Context A. Subjects in Group Conditioned Inhibition-Transfer Excitor (CI–T) were tested with compound CI–T, in which CI was the presumed conditioned inhibitor. Subjects in Group Latent Inhibition–Transfer Excitor (LI–T) were tested with compound LI–T, in which LI was the presumed latently inhibited cue. Subjects in Group Neutral Stimulus–Transfer Excitor (NS–T) were tested with compound NS–T, in which NS was associatively neutral. For all subjects, upon placement in the test chamber, time spent drinking by each subject was recorded. Immediately after completion of an initial 5 cumulative seconds of licking in the absence of any nominal stimulus, subjects were presented with the test compound; thus, all subjects were drinking at the time of test compound onset. Time to complete an additional 5 cumulative seconds of licking in the presence of the test compound was recorded. The times recorded for completion of 5 cumulative seconds of licking during the presentation of the test compound were interpreted as reflecting subjects’ expectancy of the US following onset of the test compound. The test session was 11 min in duration, and a ceiling score of 10 min was imposed on the time to complete 5 cumulative seconds of drinking in the presence of the test compound. Therefore, total exposure to the test compound was consistent across subgroups. Subjects taking more than 60 s to complete their first 5 cumulative seconds of licking were scheduled to be eliminated from the experiment for expressing abnormally high fear of the context.
Phase 2 (retardation training)
On Day 11, all subjects received reinforcement of two stimuli in Context B (i.e., a distinctly different context than the one used for LI training and CI training) during 120-min sessions. To avoid carryover effects from the summation test, the retardation training was provided only to those stimuli that were not present during the Day 10 summation test. Subjects in Group CI-T received 4 reinforced presentations each of LI, the latently inhibited cue, and NS, the neutral stimulus, interspersed. Subjects in Group LI–T received four reinforced presentations each of CI, the conditioned inhibitor, and NS, interspersed. Subjects in Group NS–T received four reinforced presentations each of CI and LI, interspersed. For all subjects, there was an ITI of 15 min between stimulus onsets. Phase 2 excitatory training was administered off baseline because the footshocks of excitatory training completely suppress baseline drinking in our preparation. All subjects also received exposure to Context A for 120 min starting approximately 2 hours after training in order to equate total exposure to the two contexts.
Test 2 (retardation assessment)
On Day 14, each subject received a retardation test in Context A. The testing procedure was the same as for the summation test, except only a single cue was presented. Subgroups CI–T LI and NS–T LI (now constituting Group LI) were tested on the LI cue. Subgroups LI–T CI and NS–T CI (now constituting Group CI) were tested on the CI cue. Subgroups CI–T NS and LI–N NS (now constituting Group NS) were tested on the NS cue.
Results and discussion
All test scores were converted to log10 seconds to better approximate the within-group normal distributions assumed by parametric statistical tests. An alpha level of .05 was adopted for all tests of statistical significance. Two subjects from each subgroup were eliminated from Experiment 1 due to an equipment malfunction during Phase 2. Additionally, two subjects from Subgroup LI–T NS and two from Subgroup NS–T CI were eliminated from this analysis due to an equipment problem that occurred during Test 1 data collection; however, they provided data for the retardation test (i.e., Test 2) as their Test 1 experience was as scheduled (i.e., only their data were lost). No subject had to be eliminated for taking over 60 s to complete the initial 5 cumulative seconds of licking. Following the loss of these subjects and data, Subgroup CI–T LI consisted of six males and four females; context was fully counterbalanced with respect to males, but females lacked two instances of the operant chamber for Test 1. Subgroup CI–T NS consisted of four males and six females; context was fully counterbalanced with respect to females, but males lacked two instances of the operant chamber for Test 1. The counterbalancing of physical stimuli serving as cues LI and NS was maintained in these two subgroups.
Subgroup LI–T CI consisted of six males and four females; context was fully counterbalanced with respect to males, but females lacked two instances of the operant chamber for Test 1. Subgroup LI–T NS consisted of four males and six females; context was fully counterbalanced with respect to females, but males lacked two instances of the operant chamber and two of Chamber R for Test 1 (also, in Test 1, this subgroup lacked two additional males). The counterbalancing of physical stimuli serving as cues CI or NS was maintained in these two subgroups.
Subgroup NS–T CI consisted of six males and fouir females; context was fully counterbalanced with respect to males, but females lacked two instances of the operant chamber and two of Chamber R for Test 1 (also, in Test 1, this subgroup lacked two additional females). Subgroup NS–T LI consisted of four males and six females; context was fully counterbalanced with respect to females, but males lacked two instances of the operant chamber for Test 1. The counterbalancing of physical stimuli serving as cues CI or LI was maintained in these two subgroups.
Examination of the data indicated that the slight disruption of the counterbalancing with respect to chamber and physical stimulus assigned to each role did not appreciably alter the results. That is, there were no appreciable differences in conditioned suppression to cues playing a given role (i.e., LI, CI, and NS) in Context A or in Context B across the different physical chambers serving as A and B or across the different physical stimuli serving as cues LI, CI, and NS.
Test 1 results (summation test)
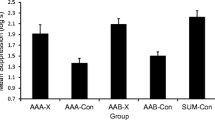
Mean conditioned suppression to the Test 1 compound CS for each group is depicted in Fig. 1. A one-way analysis of variance (ANOVA) was conducted on time to complete the first 5 cumulative seconds of licking (i.e., baseline licking prior the test cue), using the type of test compound (CI–T vs. LI–T vs. NS–T) to define conditions, which indicated no statistically significant differences among conditions, F(2, 53) = 1.20, p = .30. An analogous ANOVA was performed on time to complete 5 cumulative seconds of licking during presentation of the test compound, which found differences among test conditions, F(2, 53) = 7.89, p < .01, MSE = 0.23, Cohen’s f = 0.37, 95% CI [0.10, 0.65]. Planned comparisons were conducted using the error term from the ANOVA to test specific hypotheses. Less conditioned suppression was observed when the transfer excitor was tested in compound with the conditioned inhibitor (CI–T) than when the transfer excitor was tested in compound with either the latent inhibitor (LI–T), F(1, 53) = 11.58, p < .01, Cohen’s f = 0.45, 95% CI [0.18, 0.73], or the neutral cue (NS–T), F(1, 53) = 11.38, p < .01, Cohen’s f = 0.44, 95% CI [0.18, 0.73]. Conditioned suppression did not differ significantly between the control conditions (LI–T and NS–T), F(1, 53) < 1.
Experiment 1 results: Summation test. Mean times to complete 5 cumulative seconds of drinking in the presence of cue LI (trained as latent inhibitor), cue CI (trained as conditioned inhibitor), and cue NS (a neutral cue) in compound with cue T (a transfer conditioned excitor) on the summation test of Experiment 1. The lowest possible score was 0.7 log s. Higher scores represent stronger behavioral control by the test compound. Error bars represent standard error of the mean (see text and Table 1 for details)
Test 2 results (retardation test)
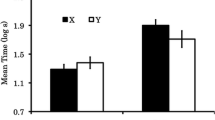
Mean conditioned suppression to the Test 2 CS for each subgroup is depicted in Fig. 2. A one-way ANOVA was conducted on the data recorded before the presentation of the test CS (i.e., baseline licking) using the retardation test stimulus (CI vs. LI vs. NS) to define conditions, which indicated no differences among conditions, F(2, 57) = 1.05, p = .35. An analogous ANOVA was performed on the data recorded during presentation of the CS, which found differences among test conditions, F(2, 57) = 14.43, p < .01, MSE = 0.12, Cohen’s f = 0.49, 95% CI [0.22. 0.76]. Planned comparisons were conducted using the error term from the ANOVA to test specific hypotheses. Greater conditioned suppression was observed to the control stimulus NS relative to both the latent inhibitor LI, F(1, 57) = 23.13, p < .01, Cohen’s f = 0.62, 95% CI [0.34, 0.90], and the conditioned inhibitor CI, F(1, 57) = 20.05, p < .01, Cohen’s f = 0.58, 95% CI [0.30, 0.85]. Suppression did not differ between cues LI and CI, F(1, 57) < 1. To assess the similarity in degree of retarded acquisition between the LI and CI cues, we conducted a Bayesian analysis, which found odds of 4.10 in favor of the null hypothesis (Rouder, Speckman, Dongchu, Morey, & Iverson 2009).
Experiment 1 results: Retardation test. Mean times to complete 5 cumulative seconds of drinking in the presence of cue LI (trained as a latent inhibitor), cue CI (trained as a conditioned inhibitor), and cue NS (an initially neutral cue) on the retardation test of Experiment 1. The lowest possible score was 0.7 log s, and the highest possible score was 2.8 log s. Higher scores represent stronger behavioral control by the test cue. Error bars represent standard error of the mean (see text and Table 1 for details)
Discussion
During the summation test, subjects received presentations of the transfer excitor (T) in compound with CI (the conditioned inhibitor) in Group CI–T, with LI (the latent inhibitor) in Group LI–T, and with NS (a cue that was associatively neutral prior to the retardation test pairings with the US) in Group NS–T. Weak behavioral control by the transfer excitor was observed in Condition CI–T relative to the other two conditions, consistent with the expectation that a conditioned inhibitor, but not a so-called]latent inhibitor would reduce conditioned suppression to a transfer excitor. On the retardation test, subjects in Groups LI and CI suppressed weakly relative to Condition NS, which is consistent with the expectation that both a latent inhibitor and a conditioned inhibitor ordinarily are retarded in acquiring behavioral control. These two tests conjointly confirm that our training of cue LI produced an effective latent inhibitor and that our training of cue CI produced an effective conditioned inhibitor (e.g., Rescorla, 1969). Critically, the level of responding did not differ appreciably between LI and CI on the retardation test. This conclusion was supported by a Bayesian analysis. Support for the null hypothesis was important because, as previously mentioned, we require similar levels of retardation for LI and CI for Experiment 2, which was designed to address the context specificity of CI training and LI training when these experiences are followed excitatory conditioning in a different context. Hence, we did, in fact, document approximately equal attenuation of excitatory responding in Context A, the context in which LI and CI were expected to be most readily expressed.
Given the identical excitatory training received by all subjects during Phase 2, the observed differences in stimulus control observed at test seemingly reflect differences in training during Phase 1. Whether these differences stem directly from what was learning in Phase 1 or indirectly from what was learned in Phase 2 as a function of prior Phase 1 training is not clear. But there is no question that stimulus control by the LI and CI cues was similar during the Test 2 of Experiment 1.
Experiment 2
In Experiment 1, approximately equal excitatory responding to the LI and CI cues was observed in Context A, the context of LI and CI training, which indicates similarly effective LI and CI training with respect to a subsequent retardation test. Having found equal response levels across these conditions when retardation testing occurred in the LI / CI treatment context, we now moved to the central focus of comparing the context specificity of LI and CI. In Experiment 2, we assessed transfer of LI and CI training to neutral Context C (and Context B). The previously reported partial context specificity of LI led us to expect some impairment of excitatory responding to the LI cue in Context C. However, the literature is less clear concerning the amount of transfer that would be seen to the CI cue in Context C.
Experiment 2 investigated the relative context specificity of associative outcome interference with excitatory training in Context B during Phase 2 following LI and CI training administered in Context A during Phase 1. Phase 1 and Phase 2 treatments were administered in different contexts in order to increase sensitivity to test context. As in Experiment 1, LI and CI cues were trained in Context A during Phase 1, followed by excitatory training of the LI and CI cues during Phase 2 in a distinctly different Context B, but in Experiment 2 behavioral testing occurred in Contexts A (Condition ABA), B (Condition ABB), and C (Condition ABC) (see Table 2). Although we continued to provide training on a potential transfer excitor so as not to deviate unnecessarily from the parameters of Experiment 1, we omitted a summation test because, as evidenced in Experiment 1, LI training does not produce a reduction responding to transfer excitors (i.e., it does not pass a summation test; Rescorla, 1971), and we were centrally interested in comparing CI and LI with respect of context specificity.
Method
Subjects
The subjects were 36 male and 36 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 183–244 g for females and 192–338 g for males. Subjects were randomly assigned to one of six groups identified by context shift design and whether testing was on the LI cue or the CI cue (ABC–CI, ABC–LI, ABB–CI, ABB–LI, ABA–CI, ABA–LI; ns = 12), counterbalanced within groups for sex. The animals were housed and maintained as in Experiment 1.
Apparatus
The apparatus was the same as in Experiment 1. Stimuli CI and LI were the Tone or SonAlert, counterbalanced within groups. Stimuli T and NS were the clicks and white noise, counterbalanced within groups but yoked to stimulus assignment of LI and CI as cues. L was the flashing light used as the excitory CS during CI training. All cues were 20 s in duration during training. The US was the same footshock that has been used in Experiment 1. The physical identities of Contexts A, B and C were counterbalanced within groups using a Latin square.
Procedure
Acclimation
On Day 1, all subjects received exposure to Context A, then to Context B, and then to Context C during three 60-min sessions. There were approximately 2 hours between acclimation sessions for a given animal. Subjects were given free access to water from lick tubes during these sessions. While in Context A, all subjects received one presentation of each cue in in the same order as in Experiment 1. The ITIs were 10 min.
Phase 1
On Days 2–6, all subjects received training in Context A equivalent to that of Phase 1(A and B) of Experiment 1. Phase 1C training of the transfer excitor T was irrelevant with respect to the current experiment and was included only to match the training regimen of Experiment 1. Subjects also received equivalent daily context exposure to Context B and Context C, during which no stimuli were scheduled to appear. These latter two sessions were separated by about 2 hours from each other and were intended to equate total exposure to Contexts A, B, and C. The order of exposure to these three contexts was the same as in acclimation and consistent across days for any given subject.
Phase 2
On Day 7, all subjects received excitatory conditioning with Stimuli CI and LI in Context B during a single 120-min session. Subjects received four reinforced presentations each of cue CI and cue LI, interspersed, with the same parameters as in Phase 2 of Experiment 1. All subjects also received equal exposure to Contexts A and C with an intersession interval of approximately 120 min and the order of exposure to the three contexts followed the same sequence as in acclimation and acquisition.
Reacclimation
On Days 8, 9, and 10, all subjects received exposure to the context in which they were scheduled to be tested in order to restore baseline drinking behavior, which might have been disrupted by the footshocks administered in Phases 1 and 2. Reacclimation exposure occurred during daily 60-min sessions during which subjects received free access to water from the lick tube, and no nominal stimuli were scheduled to appear.
Test
On Day 11, all subjects were tested for conditioned lick suppression to the test stimulus. Subjects in Groups ABC–CI and ABC–LI received presentation of Stimulus CI or LI, respectively, in Context C. Subjects in Groups ABB–CI and ABB–LI as well Groups ABA–CI and ABA–LI were tested identically except that testing occurred in Contexts B or A, respectively (see Table 2). The tests, aside from the location of testing, followed the same procedures as were used in Experiment 1.
Results and discussion
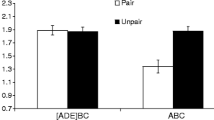
One subject from Group ABC–LI was excluded from all data analyses due to an equipment problem during testing. No subject had to be eliminated for taking more than 60 s to complete the first 5 cumulative seconds of licking. This affected the counterbalancing by removing a male that was going to be tested in the V chamber and for which the tone and SonAlert were designated as the CI and LI, respectively. Figure 3 depicts mean conditioned suppression for each group. A 2 (cue [LI vs. CI]) × 3 (test context [ABA vs. ABB vs. ABC]) factorial ANOVA performed on the time to complete the first 5 cumulative seconds of drinking (i.e., prior to CS onset) did not yield any significant main effect or interaction, all ps > .42. An analogous ANOVA performed on the time to complete 5 cumulative seconds in presence of the test cue found a significant interaction, F(2, 65) = 5.17, p < .01, MSE = 0.13, Cohen’s f = 0.36, 95% CI [0.10, 0.60]. Planned comparisons were conducted to identify the source of the interaction and test specific hypotheses. Conditioned suppression was greater to the CI cue when it was tested in Context B relative to Context A, F(1, 65) = 8.99, p < .01, Cohen’s f = 0.35, 95% CI [0.11, 0.59], which constitutes an ABA renewal-like effect for Pavlovian CI. Similarly, greater suppression was observed to the LI cue when it was tested in Context B relative to Context A, F(1, 65) = 9.04, p < .01, Cohen’s f = 0.35, 95% CI [0.11, 0.59], which constitutes an ABA renewal-like effect for LI. In Context B, cues CI and LI did not differ significantly in their control of behavior, F(1, 65) = 0.97 p = .33. More interesting are comparisons of conditioned suppression to the LI and CI cues when they were tested in the neutral context (Context C; Condition ABC) relative to when they were tested in the context in which Phase 2 excitatory training occurred (Context B; Condition ABB). The latent inhibitor, LI, yielded similarly high suppression in Contexts B and C, F(1, 65) < 1. However, greater suppression to the conditioned inhibitor, CI, was observed when it was tested in Context B, where excitatory training had occurred, relative to the neutral Context C, F(1, 65) = 13.23, p < .01, Cohen’s f = 0.42, 95% CI [0.17, 0.65]. This suggests that the influence of CI treatment transferred more readily to neutral Context C than did the influence of LI treatment.
Experiment 2 results. Mean times to complete 5 cumulative seconds of drinking in the presence of either cue CI (trained as a conditioned inhibitor) or cue LI (trained as a latent inhibitor) on the retardation test of Experiment 2. The lowest possible score was 0.7 log s, and the highest possible score was 2.8 log s. Higher scores represent stronger behavioral control by the test cue. Error bars represent standard error of the mean (see text and Table 2 for details)
These data are consistent with Sissons and Miller’s (2009) findings in which temporal contexts were manipulated. They observed that first-learned CI transferred almost completely from the temporal context of CI training (i.e., CI assessed when there was a short retention interval between training and testing, and consequently subjects were tested in a temporal context similar to that of CI training) to a distant neutral context created by a long retention interval which produced a temporal test context dissimilar to that of CI training. What is novel here is that LI was observed to be more context specific than CI despite the LI and CI treatments having been matched in producing retardation of excitatory responding when testing occurred in Context A in both the present experiment and Experiment 1. This conclusion is congruent with Miller et al. (2015, Experiment 1), although that design did not use a change of context between LI and excitatory training nor did it compare LI directly with CI. Notably, the observed strong responding to the LI cue in Context C (i.e., lack of transfer of any detectable LI effect to Context C) is not fully consistent with prior reports of LI partially transferring to a neutral test context (e.g., Swartzentruber & Bouton, 1992; Westbrook et al. 2000); however, the procedures and parameters of the present experiment differed appreciably from those prior studies. In the present experiment, using behaviorally matched LI and CI training procedures, CI training more readily transferred to Context C than did LI. To the best of our knowledge, this strong transfer of first-learned CI training to a neutral test context following excitation training is a novel observation. This new finding, despite comparable weak suppression to Cues CI and LI in Context A and comparable strong suppression in Context B, suggests that CI training is much less context specific than LI training. Importantly, the low responding to Cues LI and CI in Context A and high responding to these cues in Context B were quite similar and not likely an artifact of floor or ceiling effects in that none of the group means approached the floor or ceiling values imposed by our procedure (see Fig. 3). However, one might argue that a behavioral floor effect could mask actual differences in the effectiveness of the CI and LI treatments. That possibility is assessed in Experiment 3.
Experiment 3
The data from Experiment 2 support the view that CI training transfers more strongly to a neutral context than does LI training. However, an alternative explanation of the results of Experiment 2 is that the memory of CI training may have been stronger than the memory of LI training, thereby allowing CI training to generalize more between contexts than LI training. We had attempted to preclude such an account by matching the degree of conditioned suppression to the CI and LI cues in Experiment 1. Although we were successful in this endeavor, the claim that LI and CI learning were matched with respect to testing in the Phase 1 context might be met with some skepticism and should not rest on a mere null result. Hence, we cannot definitively reject the possibility that the critical results of Experiment 2 were due to simple differences in the strengths of CI and LI training. Toward assessing this possibility, in Experiment 3 we sought to increase the strength of LI training to determine whether that would increase transfer of its effect to Context C. Analogously, overtraining in Phase 2 has been reported to facilitate transfer of Phase 2 training to a neutral context in other instances of two-phase retroactive interference. For example, large numbers of extinction trials have been reported to increase contextual transfer of nonreinforcement treatment (i.e., reduce the magnitude of ABC renewal; Denniston, Chang, & Miller, 2003; Laborda & Miller, 2013; Thomas, Vurbic, & Novak, 2009). In Experiment 3, all subjects received LI training in Phase 1 in Context A (120 presentations in Condition Few; 1,200 presentations in Condition Many), and then reinforcement of the LI cue in Phase 2 in Context B. Note that the 120 presentations in Condition Few is the same number used in Experiments 1 and 2. Retardation testing took place in either Context A (Group Many LI–ABA), Context B (Groups Few LI–ABB and Many LI–ABB), or Context C (Groups Few LI–ABC and Many LI–ABC). Critically, we were interested in whether many preexposure trials would reduce the context specificity of LI training that we observed in Experiment 2 when it was tested in Context C relative to Context B (see Table 3). Group Many LI–ABA was of included because it provided a measure of the efficacy of our extensive LI training in the preexposure context.
If the greater context specificity of LI training relative to CI training observed in Experiment 2 was simply due to insufficient LI training, then appreciably increasing the number of LI-preexposure trials (1,200) should result in less context specificity of LI. That is, we would expect greater generalization of LI training to Context C, which would take the form of less conditioned suppression to LI on a retardation test after 1,200 preexposure trials than after only 120 trials.
Method
Subjects
The subjects were 30 male and 30 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 195–377 g for males and 160–376 g for females. Subjects were randomly assigned to one of five groups (Few LI–ABC, Few LI–ABB, Many LI–ABC, Many LI–ABB, Many LI–ABA; ns = 12), counterbalanced within groups for sex. The animals were housed and maintained as in Experiments 1 and 2.
Apparatus
The apparatus was the same as in Experiments 1 and 2. Stimulus LI was the compound tone or SonAlert, counterbalanced within groups, and was 20 s in duration during both phases of training. The US was the same footshock as had been used in the prior experiments. The physical identities of Contexts A, B, and C were counterbalanced within groups using a Latin square.
Procedure
Acclimation
On Day 1, all subjects received exposure to Context A, then to Context B, and then to Context C during three 60-min sessions. There was 1 hour between acclimation sessions. Subjects were given free access to water from lick tubes on these days.
Phase 1
On Days 2–6, all subjects received LI training in Context A. On each day, subjects in Condition Few–LI received 24 nonreinforced presentations of cue LI in a daily 24-min session (mean ITI = 20 s). Subjects in Condition Many–LI received 240 nonreinforced presentations of cue LI in a daily 240-min session (mean ITI = 20 s). Subjects also received exposure to Context B and Context C equal in duration to Context A, with an intersession interval of approximately 4 hr, during which no nominal stimulus was presented. Order of exposure to these three contexts was the same as in the acclimation procedure of the current experiment and as in the analogous phase in Experiment 2. We manipulated the number of trials across conditions while maintaining the same ITIs between the Few and Many conditions. However, due to available resources, we reduced the ITIs relative to the previous experiments from 100 s to 20 s. Although any deviation limits comparison across experiments, this particular change, if anything, would be expected to create a bias toward stronger LI (e.g., Escobar, Arcediano, & Miller, 2002). This would presumably be a bias toward LI being more generalizable, which is contrary to what was actually observed in this experiment (see Results).
Phase 2
On Day 7, all subjects received four reinforced presentations of the LI cue in Context B during a 60-min session. There was an ITI of 12 min between stimulus onsets. All subjects also received exposure to Context B and Context C (12 min in the Few condition, 120 min in the Many condition) with an intersession interval of approximately 2 hr. Order of exposure to these three contexts was the same as in the previous phases and in Experiment 2.
Reacclimation
On Days 8–10, all subjects received 60 min of exposure to each of Contexts A, B, and C to restore baseline drinking behavior, which might have been disrupted by the footshocks. Subjects received free access to water, and no nominal stimulus was scheduled to appear. Order of exposure to the three contexts was the same as in the previous phases and in Experiment 2.
Test
On Day 11, all subjects were tested for conditioned lick suppression to cue LI. Subjects in Condition ABC received presentation of Cue LI in Context C. Subjects in Condition ABB received presentation of Cue LI in Context B. Subjects in Condition ABA received presentation of Cue LI in Context A. Testing followed the same procedure as in Experiment 2.
Results and discussion
Group means for conditioned suppression to Cue LI are represented in Fig. 4. Eight subjects (four from Group Few LI–ABC and four from Group Few LI–ABB) were eliminated from the experiment due to an experimenter error during Phase 1. No subject had to be eliminated for taking more than 60 s to complete the first 5 cumulative seconds of licking on the test trial. As a result of subject loss, Group Few LI–ABB consisted of four males and four females, and the LI was more frequently the tone for the females and the SonAlert for the males. Group Few LI–ABC consisted of four males and four females, and the LI was more frequently the tone for the males and the SonAlert for the females.
Experiment 3 results. Mean times to complete 5 cumulative seconds of drinking in the presence of cue LI (trained as a latent inhibitor) on the retardation test of Experiment 3. The lowest possible score was 0.7 log s and the highest possible score was 2.8 log s. Higher scores represent stronger behavioral control by the test cue. Error bars represent standard error of the mean (see text and Table 3 for details)
A one-way ANOVA performed on the time to complete the first 5 cumulative seconds of licking in the context (i.e., baseline licking prior to CS onset) yielded no significant differences among the groups, F(4, 47) = 1.80, p = .14. A similar ANOVA conducted on time to complete 5 s of cumulative drinking in the presence of the CS detected differences among groups, F(4, 47) = 6.52, p < .01, MSE = 0.26, Cohen’s f = 0.64, 95% CI [0.32, 0.96]. Planned comparisons using the error term from the ANOVA were conducted to identify the sources of the differences. A comparison of Group Few LI–ABB and Group Few LI–ABC found when few LI preexposure trials were administered, no significant difference in stimulus control occurred between testing in the context of excitatory conditioning (B) and a neutral context (C), F(1, 47) < 1, which is consistent with the results of Experiment 2. Group Many LI–ABA responded less than Groups Many LI–ABB, F(1, 47) = 19.00, p < .01, Cohen’s f = 0.60, 95% CI [0.30, 0.90], and Many LI–ABC, F(1, 47) = 18.70, p < .01, Cohen’s f = 0.59, 95% CI [0.30, 0.89], which indicates that testing in the context of LI training (A) reduced responding relative to testing in the context of excitatory acquisition (B) or in a neutral context (C) when many LI trials were given. Critically, no difference was observed between testing in the context of excitatory conditioning and testing in an irrelevant context when many LI trials were given (Group Many LI–ABB vs. Group Many LI–ABC), F(1, 47) < 1 (see Fig. 4).
These results indicate that even with 10 times the number of LI preexposure trials that was administered in Experiment 2 (and the Few condition of Experiment 3), LI remains largely context specific and does not transfer well to a neutral context. This is in contrast to CI, which did transfer to a neutral context in Experiment 2. Finally, a 2 (number of LI trials: Few vs. Many) × 2 (context of testing: ABB vs. ABC) factorial ANOVA yielded no significant main effect of number of LI trials, F(1, 36) = 1.73, p = .19, no main effect of context of testing, F(1, 36) < 1, and no interaction, F(1, 36) < 1. The lack of a significant effect of Number of LI trials suggests that 1,200 preexposure trials in the present preparation did not produce appreciably more of an LI effect than did 120 preexposure trials. Nevertheless, this lack of differences could be due the loss of eight subjects in Condition Few (four per group). In order to achieve a significant main effect of number of trials given our current effect size (Cohen’s f = .135), the estimated required total sample size (based on power = 0.8) for the four groups in the 2 × 2 ANOVA would have to be 433, which is far larger than the usual and reasonable sample size in this type of experiments. Thus, the LI effect appears to have been nearly asymptotic after 120 preexposure trials.
Overall, the results of the current experiment suggest that the observed difference in context specificity between CI and LI in Experiment 2 did not arise from an inadequate amount of LI training. A much higher number of preexposure trials (1,200) did not decrease the context specificity of LI training. Thus, the greater context specificity of LI relative to CI observed in Experiment 2 does not seem to be a consequence of an insufficient number of LI preexposure trials.
General discussion
The present experiments collectively examined the context specificity of proactive outcome interference produced by LI training or CI training in Phase 1 and conditioned excitation training in Phase 2. In Experiment 1, we demonstrated that our procedures and parameters successfully produced both a Pavlovian conditioned inhibitor and a latently inhibited cue in a single experiment, as indicated by a summation test and a retardation test (Rescorla, 1971). In Experiment 2, we directly compared the context specificity of proactive interference of LI training and CI training with subsequent excitatory conditioning. After LI training and CI training during Phase 1 in Context A and excitatory training during Phase 2 in Context B, subjects were tested in either Context A, B, or C. More proactive interference (i.e., more retardation of excitatory responding) was observed with both the CI and LI cues when testing occurred in the context in which the LI and CI training had occurred (Context A) than in the context in which the excitatory conditioning had occurred (Context B). Thus, not surprisingly, after ambiguous training in two different contexts (i.e., a different outcome in each of the two training contexts), testing in either of these contexts resulted in behavior consistent with the training that had previously occurred in that context.
Of central interest was what happened when subjects were tested in an associatively neutral context (C). Pavlovian inhibition training produced strong transfer of the first-learned inhibitory-like memory to this neutral context. That is, we observed weak stimulus control of behavior (little conditioned suppression during the retardation test) to the CI cue in Context C. This contrasts with the strong behavioral control (expression of Phase 2 excitatory memory) by the LI cue that was observed in Context C. Thus, the results of Experiment 2 support the view that LI training in Phase 1 transfers weakly to a neutral context relative to CI training in Phase 1. Alternatively stated, it appears as if LI training is decidedly more context specific than is CI training that precedes excitatory conditioning. Notably, the tendency of CI training to generalize across test contexts seems to be great when it precedes excitatory training (i.e., is first learned). But as mentioned above, prior reports indicate that when CI training follows excitatory conditioning (i.e., is second learned), CI training is seemingly relatively context specific (e.g., Nelson, 2002; Sissons & Miller, 2009). Experiment 3 was designed to assess the possibility that the observed difference in transfer between CI and LI to neutral Context C was a product of differing strengths of the inhibitory-like memories. We found that a substantially larger number of CS preexposure trials (1,200) did not enhance transfer of the LI effect to the neutral test context. This suggests that the difference in context specificity between CI and LI observed in Experiment 2 did not arise from a weak LI effect relative to that of CI.
It is worth noting that the present research did not include a group that received simple excitatory training without either prior LI or CI training. However, our central concern here was relative degrees of context specificity of CI and LI across contexts rather than absolute amount of CI or LI.
Having discounted accounts of the observed difference in context specificity of CI and LI based on mere differences in strength of learning, the difference in context specificity may be viewed as indicative of there being (at least) two distinctly different types of memories of nonreinforcement, a view which is congruent with the observation that a conditioned inhibitor passes a negative summation test when presented in compound with a transfer excitor, whereas a latent inhibitor does not (the present Experiment 1; Reiss & Wagner, 1972; Rescorla, 1971). Latent inhibition treatment may reduce responding on a retardation test because it reduces the salience of the stimulus. However, reduced attention should not interfere with the excitation of a second cue on a summation test. Thus, attentional models can explain the observations in Experiment 1. But they cannot explain why in Experiment 2 stronger responding to cue LI than cue CI was observed in Context C. Yet cues LI and CI yielded similar degrees of weak suppression in Context A and similar degrees of strong suppression in Context B.
The absence of appreciable transfer of LI training to Context C, despite evidence in Context A that memory of the LI training had survived Phase 2 excitatory training, is congruent with Miller et al. (2015) but appears contrary to earlier data reported by Swartzentruber and Bouton (1992) and Westbrook et al. (2000), both of whom observed partial transfer of LI training to a neutral context. Swartzentruber and Bouton observed less responding to a preexposed cue when subsequent training and testing took place in different contexts; however, their LI cue still exhibited some degree of behavioral control. As their design did not include a test of the LI cue back in the context of preexposure, they could not assess how much of what was learned in the preexposure context failed to transfer to the third context. Regarding Westbrook et al., they did observe less responding in a third context relative to the context of excitatory training, and also, they observed more responding in the third context relative to the context of preexposure. In our experiments, we replicated the difference between the contexts of preexposure and excitatory training. Granted, in Experiment 2, we did not observe a significant difference in responding to cue LI in the third context (C) relative to the excitation context (B). However, suppression in the excitation training context was high in that experiment, which may have masked any potential differences. Therefore, it is a plausible that such a difference may be found with other parameters, and we simply failed to observe it due to insensitivity of our measure. Nevertheless, our conclusions still stand; CI treatment generalized more to a third context than did LI treatment.
Presumably, procedural differences between the present experiments and these prior studies were the basis of the weaker transfer of LI training that we observed. Beyond procedural concerns, these prior reports did not examine contextual transfer of CI training with procedures matched to those of LI training. Thus, there are no data contrary to our observation that transfer of first-learned CI training was stronger than transfer of LI training. We conclude that given matched conditions, CI training transferred better to Context C than did LI training. Importantly, we are not suggesting that transfer of LI (or CI) training between contexts is all or none. Transfer is graded. We are only asserting that CI training transfers better to a third context than does LI training when the treatments are selected to yield similarly weak behavioral control in the inhibitory training context (A) and similarly strong behavioral control in the excitatory training context (B). Notably, Experiment 2 is the first demonstration of CI having better transfer than LI when LI and CI training were demonstrably equated behavior in Contexts A and B.
The results of Experiment 2 might be viewed as speaking more generally to the context specificity of outcome interference. Excitatory conditioning following CI training did not appreciably transfer to a neutral context (C). This is an instance of excitatory second-learned information being context specific, whereas the generalization of conditioned excitation to Context C following LI training is an instance of excitatory second-learned information not being context specific. Bouton (1993), in his analysis of retroactive interference, provided two accounts of why inhibitory-like information (such as the learning acquired during extinction treatment following excitatory conditioning) appears to be context specific. The first account suggests that context specificity is an inherent property of inhibitory-like associations. The second account suggests that second-learned information, regardless of whether it is inhibitory or excitatory, will be context specific. As these two accounts are perfectly confounded when applied to extinction (i.e., they cannot be dissociated), efforts to differentiate between them have focused on nonreinforcement preceding reinforcement (i.e., LI). This creates an instance of proactive interference in which excitatory conditioning follows the inhibitory-like treatment. Prior contrasts between these two accounts have rarely examined more than one type of inhibitory-like training in a single experiment with matched conditions. Our Experiment 2 provides new evidence to inform accounts of context specificity for inhibitory-like associations.
In Experiment 2, one or both of the training contexts (A or B) presumably became a discriminative stimulus that facilitated later retrieval of information acquired in that context. Consistent with this expectation, testing in the context in which inhibitory training occurred evidenced more expression of inhibitory learning, whereas testing in the context of excitatory training evidenced more expression of excitatory learning. Presumably, one or both of these contexts acted as discriminative stimulus (i.e., an occasion setter; e.g., Holland, 1983, 1989a, b; Miller & Oberling, 1998; Rescorla, 1985), thereby facilitating the expression of the memories acquired in that context. Whether the context of inhibitory-like training (Context A) or excitatory training (Context B) was the more effective occasion setter can best be determined by examining behavioral control in a neutral context (C). If the context of excitatory training (B) was an occasion setter, excitatory training should not transfer to Context C, and if the context of inhibitory-like training (A) was an occasion setter, inhibitory-like training should not transfer to Context C. In fact, we observed little transfer to Context C after LI training (i.e., strong suppression to the target cue was observed). This suggests that the context of LI training acted like a negative occasion setter, which made the LI effect specific to the context in which LI training occurred. In contrast, strong transfer of CI training to Context C was observed (i.e., weak suppression to the target cue), suggesting that the context of subsequent excitatory training acted like a positive occasion setter, effectively allowing the first-learned CI training to generalize readily across contexts. This analysis reduces to what was previously stated: When inhibitory-like training occurred in Phase 1 and excitatory training occurred in Phase 2, greater context specificity of the inhibitory-like training was seen with LI training than with CI training. This points to different mechanisms underlying these two outcome interference phenomena.
The present research documents for the first time in a single experiment appreciable difference in context specificity between LI and CI training. But it does not explain the basis for this difference, nor was it intended to do so. That task must be addressed in future research; however, we feel that some speculation would be appropriate at this point. Notably, Pavlovian CI training involves presentation of the target cue in compound with an excitatory CS that creates an expectation of the US which is unfulfilled (i.e., there is a violation of US expectancy), whereas LI training involves presentation of the target cue in the absence of any expectation of the US (i.e., there is no violation of US expectancy; but see Hall & Rodriguez, 2010, who assume that a novel stimulus such as the one used in LI training carries some inherent expectation that something significant may occur). This account is congruent with the data, but it only relocates the question. One must now ask why the presence or absence of US expectation during first-learned inhibitory-like training influences the context specificity of the memory established during this training. One possibility is that the violation of US expectation in CI training might be expected to enhance learning (e.g., Rescorla & Wagner, 1972), but even in this framework enhancement of learning alone should not directly influence context specificity of whatever was learned. Moreover, the observed difference in context specificity of CI and LI does not appear to be a product of stronger training in one situation than the other for two reasons. First, in Experiment 1, equal retardation resulted from CI and LI training with the present parameters, and second, after we administered additional LI training in Experiment 3, LI training still proved to be highly context specific.
There are at least two mechanisms that potentially could explain the observed difference in context specificity. One possible family of accounts depends directly on a difference in attention between conditioned inhibitors and latent inhibitors. Upon first consideration, one might expect subjects to pay more attention to a conditioned inhibitor than a latent inhibitor because only the former has been paired with a presumably attention-focusing biologically significant cue (i.e., a cue which has been paired with a US). This position is consistent with several well-known models (e.g., Lubow, 1989; Mackintosh, 1975) that emphasize conditioned changes in attention (or at least associability). This leads to the expectation that the informational basis of CI should be acquired faster than that of LI, but does not speak to why LI is more context specific than is CI. Other associative accounts that rely on the concept of attention predict a loss of attention with both a latent inhibitor and a conditioned inhibitor (e.g., Pearce & Hall, 1980). Nevertheless, an attentional account of the LI effect (e.g., Lubow, 1989) assumes that LI training prevents the acquisition of the second-learned training, which is contrary to the recovery of responding observed in Group ABB–LI relative to Group ABA–LI in Experiment 2. Instead, our data seem to be better explained in terms of retrieval processes that operate at the time of testing.
Adding a bit more nuance to an attentional account begins to speak to the observed difference in context specificity. During CI training, the target cue (CI) is paired with an excitatory CS (L in the notation of the present report). The acquired biological significance of the excitatory CS should attract attention to itself as well as the conditioned inhibitor (CI) due to the CI’s high degree of contiguity with L. Thus, the excitatory CS should distract the subject from attending to the training context. Consequently, relatively little learning about the context should occur during CI training. Granted, when CI training occurs after excitatory training (Nelson, 2002; Sissons & Miller, 2009), the additional ambiguity of the CI may encourage greater attention to the context to resolve this ambiguity than when CI is first learned (see Rosas & Callejas-Aguilera, 2007; Rosas, García-Gutiérrez, & Callejas-Aguilera, 2006, for elaboration on the effects of ambiguous experience on attention to the context). In contrast, during LI training, no biologically significant cue is present to distract the subject from attending to the context. Consequently, the context is likely able to play a larger role in informing behavioral expression of what was learned in that phase.
This emphasis on differential strength of LI–context associations is consistent with several different accounts of LI that hinge on cue–context associations (e.g., Miller & Matzel, 1988; Wagner, 1981), all of which do well explaining the high context specificity of LI relative to CI. Although the details of the explanation of differential context specificity differs appreciably across these models, they agree in positing that at least part of the response deficit seen following LI training and subsequent excitatory training is due to a failure to express information that was acquired during the target cue–US excitatory training that constitutes a retardation test. This contrasts markedly with other accounts of LI that posit a decrease in attention (or associability) attenuates acquisition of the cue–US association during the excitatory condition in Phase 2 (e.g., Lubow, 1989; Pearce & Hall, 1980). These attentional models have greater difficulty in explaining the high context specificity of LI because they view the response deficit produced by LI training as arising entirely from a failure to acquire a cue–US association during the retardation test. If learning does not occur, responding to the LI cue should not be observed even when the target cue is tested outside the context of LI training.
Additional research will be needed to test these possibilities. But whatever the underlying basis of this difference in context specificity between CI and LI, identification of this difference resolves a seeming discrepancy in the literature concerning the context specificity of inhibitory-like information in proactive interference situations. Moreover, the present observation that later expression of excitatory conditioning trained during Phase 2 was modulated by the test context indicates that memories of training in each phase of training were formed and retained. As previously stated, this finding challenges accounts of LI and CI that posit that the observed retardation in development of stimulus control by the target cue is due to a failure to learn (e.g., the view that LI training decreases attention to the target cue, thereby retarding excitatory acquisition per se). Along with other reports (e.g., Miguez, Soares, & Miller, 2015), the present experiments indicate that the associative interference that produces retarded acquisition of behavioral control is due to conflict at test between memories of the two training experiences, not the product of an acquisition or retention deficit.
References
Bouton, M. E. (1993). Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin, 114, 80–99. doi:https://doi.org/10.1037/0033-2909.114.1.80
Bouton, M. E., & Bolles, R. C. (1979). Contextual control of the extinction of conditioned fear. Learning and Motivation, 10, 445–466. doi:https://doi.org/10.1016/0023-9690(79)90057-2
Channell, S., & Hall, G. (1983). Contextual effects in latent inhibition with an appetitive conditioning procedure. Animal Learning & Behavior, 11, 67–74. doi:https://doi.org/10.3758/BF03212309
Denniston, J. C., Chang, R. C., & Miller, R. R. (2003). Massive extinction treatment attenuates the renewal effect. Learning and Motivation, 34, 68–86. doi:https://doi.org/10.1016/S0023-9690(02)00508-8
Escobar, M., Arcediano, F., & Miller, R.R. (2002). Latent inhibition and contextual associations. Journal of Experimental Psychology: Animal Behavior Processes, 28, 123–136. doi:https://doi.org/10.1037/0097-7403.28.2.123
Hall, G., & Channell, S. (1985). Differential effects of contextual change on latent inhibition and on the habituation of an orienting response. Journal of Experimental Psychology: Animal Behavior Processes, 11, 470–481. doi:https://doi.org/10.1037/0097-7403.11.3.470
Hall, G., & Rodriguez, G. (2010). Associative and nonassociative processes in latent inhibition: An elaboration of the Pearce-Hall model. In R. E. Lubow & I. Weiner (Eds.), Latent inhibition: Cognition, neuroscience and applications to schizophrenia (pp. 114–136). New York: Cambridge University Press. doi:https://doi.org/10.1017/CBO9780511730184.007
Hearst, E. (1972). Some persistent problems in the analysis of conditioned inhibition. In R. A. Boakes & M. S. Halliday (Eds.), Inhibition and learning (pp. 5–39). London: Academic Press.
Holland, P. C. (1983). Representation-mediated overshadowing and potentiation of conditioned aversions. Journal of Experimental Psychology: Animal Behavior Processes, 9, 1–13. doi:https://doi.org/10.1037/0097-7403.9.1.1
Holland, P. C. (1989a). Acquisition and transfer of conditional discrimination performance. Journal of Experimental Psychology: Animal Behavior Processes, 15, 154–165. doi:https://doi.org/10.1037/0097-7403.15.2.154
Holland, P. C. (1989b). Occasion setting with simultaneous compounds in rats. Journal of Experimental Psychology: Animal Behavior Processes, 15, 183–193. doi:https://doi.org/10.1037/0097-7403.15.3.183
Laborda, M. A., & Miller, R. R. (2013). Preventing return of fear in an animal model of anxiety: Additive effects of massive extinction and extinction in multiple contexts. Behavior Therapy, 44, 249–261. doi:https://doi.org/10.1016/j.beth.2012.11.001
Lorden, J. F., Rickert, E. J., & Berry, D. W. (1983). Forebrain monoamines and associative learning: I. Latent inhibition and conditioned inhibition. Behavioural Brain Research, 9, 181–199. doi:https://doi.org/10.1016/0166-4328(83)90127-4
Lovibond, P. F., Preston, G. C., & Mackintosh, N. J. (1984). Context specificity of conditioning, extinction, and latent inhibition. Journal of Experimental Psychology: Animal Behavior Processes, 10, 360–375. doi:https://doi.org/10.1037/0097-7403.10.3.360
Lubow, R. E. (1989). Latent inhibition and conditioned attention theory. Cambridge: Cambridge University Press. doi:https://doi.org/10.1017/CBO9780511529849
Lubow, R. E., & Moore, A. U. (1959). Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology, 52, 415–419. doi:https://doi.org/10.1037/h0046700
Mackintosh, N. J. (1975). A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review, 82, 276–298. doi:https://doi.org/10.1037/h0076778
Miguez, G., Soares, J. S., & Miller, R. R. (2015). The role of test context in latent inhibition of conditioned inhibition: Part of a search for general principles of associative interference. Learning & Behavior, 43(3), 228–242. doi:https://doi.org/10.3758/s13420-015-0175-0
Miller, R. R., & Matzel, L. D. (1988). The comparator hypothesis: A response rule for the expression of associations. In G. H. Bower (Ed.), The psychology of learning and motivation: Advances in research and theory (Vol. 22, pp. 51–92). San Diego, CA: Academic Press.
Miller, R. R., & Oberling, P. (1998). Analogies between occasion setting and Pavlovian conditioning. In N. A. Schmajuk & P. C. Holland (Eds.), Occasion setting: Associative learning and cognition in animals (pp. 3–35). Washington, DC: American Psychological Association. doi:https://doi.org/10.1037/10298-001
Miller, R. R., Laborda, M. A., Polack, C. W., & Miguez, G. (2015). Comparing context specificity of extinction and latent inhibition. Learning & Behavior, 43, 384–395. doi:https://doi.org/10.3758/s13420-015-0186-x
Nelson, J. (2002). Context specificity of excitation and inhibition in ambiguous stimuli. Learning & Motivation, 33, 284–310. doi:https://doi.org/10.1006/lmot.2001.1112
Pavlov, I. P. (1927). Conditioned reflexes (G.V. Anrep, Ed. & Trans.). London: Oxford University Press.
Pearce, J. M., & Hall, G. (1980). A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review, 87, 532–552. doi:https://doi.org/10.1037/0033-295X.87.6.532
Polack, C. W., Laborda, M. A., & Miller, R. R. (2013). On the differences in degree of renewal produced by the different renewal designs. Behavioural Processes, 99, 112–120. doi:https://doi.org/10.1016/j.beproc.2013.07.006
Polack, C. W., Jozefowiez, J., & Miller, R. R. (2017). Stepping back from ‘persistence and relapse’ to see the forest: Associative interference. Behavioural Processes. Advance online publication. doi:https://doi.org/10.1016/j.beproc.2017.03.014
Reiss, S., & Wagner, A. R. (1972). CS habituation produces a ‘latent inhibition effect’ but no active ‘conditioned inhibition’. Learning and Motivation, 3, 237–245. doi:https://doi.org/10.1016/0023-9690(72)90020-3
Rescorla, R. A. (1969). Pavlovian conditioned inhibition. Psychological Bulletin, 72, 77–94. doi:https://doi.org/10.1037/h0027760
Rescorla, R. A. (1971). Summation and retardation tests of latent inhibition. Journal of Comparative and Physiological Psychology, 75, 77–81. doi:https://doi.org/10.1037/h0030694
Rescorla, R. A. (1985). Associationism in animal learning. In L. Nilsson & T. Archer (Eds.), Perspectives on learning and memory (pp. 39–61). Hillsdale: Erlbaum.
Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York: Appleton-Century-Crofts.
Rosas, J. M. & Callejas-Aguilera, J. E. (2007). Acquisition of a conditioned taste aversion becomes context dependent when it is learned after extinction. Quarterly Journal of Experimental Psychology, 60, 9–15. doi:https://doi.org/10.1080/17470210600971519
Rosas, J. M., García-Gutiérrez, A., & Callejas-Aguilera, J. E. (2006). Effects of context change upon retrieval of first and second learned information in human predictive learning. Psicológica, 27, 35–56.
Rouder, J., Speckman, P., Dongchu S., Morey, R., & Iverson, G. (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. doi:https://doi.org/10.3758/PBR.16.2.225
Sissons, H. T., & Miller, R. R. (2009). Spontaneous recovery of excitation and inhibition. Journal of Experimental Psychology: Animal Behavior Processes, 35, 419–426. doi:https://doi.org/10.1037/a0014815
Swartzentruber, D., & Bouton, M. E. (1986). Contextual control of negative transfer produced by prior CS–US pairings. Learning and Motivation, 17, 366–385. doi:https://doi.org/10.1016/0023-9690(86)90004-4
Swartzentruber, D., & Bouton, M. E. (1992). Context sensitivity of conditioned suppression following preexposure to the conditioned stimulus. Animal Learning & Behavior, 20, 97–103.
Thomas, B. L., Vurbic, D., & Novak, C. (2009). Extensive extinction in multiple contexts eliminates the renewal of conditioned fear in rats. Learning and Motivation, 40, 147–159. doi:https://doi.org/10.1016/j.lmot.2008.10.002
Wagner, A. R. (1981). SOP: A model of automatic memory processing in animal behavior. In N. E. Spear & R. R. Miller (Eds.), Information processing in animals: Memory mechanisms (pp. 5–47). Hillsdale, NJ: Erlbaum.
Westbrook, R., Jones, M. L., Bailey, G. K., & Harris, J. A. (2000). Contextual control over conditioned responding in a latent inhibition paradigm. Journal of Experimental Psychology: Animal Behavior Processes, 26, 157–173. doi:https://doi.org/10.1037/0097-7403.26.2.157
Acknowledgements
This research was supported by National Institute of Mental Health Grant 33881. The authors thank Vincent DeNinis, Sara O’Hara, and Julia Soares for their comments on an earlier version of this manuscript. Gonzalo Miguez was partially supported by the Fulbright Program and FONDECYT #1160132 and PAI #79140028, both from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT-Chile). Questions concerning this research should be addressed to Ralph R. Miller, Department of Psychology, SUNY-Binghamton, Binghamton, NY 13902-6000, USA; e-mail: rmiller@binghamton.edu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miguez, G., McConnell, B., Polack, C.W. et al. Proactive interference by cues presented without outcomes: Differences in context specificity of latent inhibition and conditioned inhibition. Learn Behav 46, 265–280 (2018). https://doi.org/10.3758/s13420-017-0306-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-017-0306-x