Abstract
Most adolescent risk taking occurs in the presence of peers. Prior research suggests that peers alter adolescents’ decision making by increasing reward sensitivity and the engagement of regions involved in the processing of rewards, primarily the striatum. However, the potential influence of peers on the capacity for impulse control, and the associated recruitment of the brain’s control circuitry, has not yet been adequately examined. In the current study, adolescents underwent functional neuroimaging while they completed interleaved rounds of risk-taking and response-inhibition tasks. Social context was manipulated such that the participants believed they were either playing alone and unobserved, or watched by an anonymous peer. Compared to those who completed the tasks alone, adolescents in the peer condition took more risks during the risk-taking task and exhibited relatively heightened activation of the striatum. Activity within this striatal region also predicted individual differences in overall risk taking. In contrast, the presence of peers had no effect on behavioral response inhibition and had minimal impact on the engagement of typical cognitive control regions. In a subregion of the anterior insula engaged mutually by both tasks, activity was again found to be sensitive to social context during the risk-taking task, but not during the response-inhibition task. These findings extend the evidence that the presence of peers biases adolescents towards risk taking by increasing reward sensitivity rather than disrupting cognitive control.
Similar content being viewed by others
Adolescents are often characterized by their greater tendency to engage in risky behaviors relative to children and adults. Increases in risk-taking during adolescence are linked to a series of biological, psychological, and social changes that occur throughout this period of life (for review see, Steinberg, 2010). One of the most prominent social changes that impacts adolescents’ involvement in risk taking is the significant increase in the amount of time spent with peers. Epidemiological studies have shown that the majority of risky behaviors during adolescence occur in peer groups, an effect not seen among children or adults (for review see, Albert Chein & Steinberg, 2013). The prevalence of risky behaviors among groups of adolescents has made peer influences an important focus of studies aimed at understanding adolescent risk taking.
One popular explanation for increased risk-taking among adolescents in groups is that peers explicitly encourage each other to behave recklessly. However, recent experiments demonstrate that adolescents take more risks when they merely believe that a peer is watching them from another room (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Smith, Chein, & Steinberg, 2014a), in the complete absence of any explicit encouragement from the peer(s) to engage in risk-taking. Thus, although some real-world risk-taking in peer groups is undoubtedly due to the specific content of interactions with peers, it is clear that explicit peer pressure is only a part of the phenomenon (e.g., Chein et al., 2011; Smith et al., 2014b). Accordingly, researchers have begun exploring other mechanisms that may contribute to adolescents’ susceptibility to peer influence during decision-making.
In the present study, we extend this body of work by investigating whether peers impact the processes that support self-regulation, the processes that support the evaluation of rewards, or both. This work is guided by a class of developmental theories, known as “dual systems” or “maturational imbalance” models (Casey, Getz, & Galván, 2008; Luna & Wright, 2016; Steinberg, 2008), that have provided a helpful framework for understanding why adolescents are more likely than other age groups to engage in risky behaviors, and how peers may influence the decision-making process. While there have been substantive critiques of this basic neurodevelopmental framework (see, Crone & Dahl, 2012; Ernst, 2015; Pfeiffer & Allen, 2012), and while the framework may not adequately capture all of the complex and interactive processes that underlie adolescent decision making, it is a prominent model in the field that has usefully guided work in our lab and others.
Within these models, the asynchronous development of sensation seeking—the tendency to seek varied, novel, complex, and intense sensations and experiences (Zuckerman, 1994)—and self regulation—the deliberate modulation of one’s thoughts, feelings, or actions in the pursuit of planned goals (Smith, Chein, & Steinberg, 2013)—creates a window during adolescence wherein reward-seeking behaviors are favored. These models are rooted in evidence indicating a reorganization of neural regions and networks that occurs across adolescence (Casey, 2015). Major changes within the brain’s reward-processing circuitry, which includes the striatum, occur early in adolescence, around the time of puberty, and are hypothesized to evoke an increase in reward sensitivity that results in more frequent sensation seeking (for review, see, Galván, 2010). These changes in the reward-processing system are thought to occur as brain regions involved in self regulation and response inhibition (i.e., the cognitive control system) mature along a slower trajectory of development from preadolescence into young adulthood (Luciana & Collins, 2012; Luna & Wright, 2016; Shulman et al., 2016). Critically, the protracted maturation of the cognitive control system may limit an adolescent’s ability to exert top-down control over an especially reactive reward-processing system during decision making, thus increasing the likelihood that the adolescent will select affectively-driven behaviors, such as risk taking.
Within the dual systems framework, there are potentially different ways in which adolescents’ peers may sway the decision-making process to favor risky behaviors. Specifically, the presence of peers could exaggerate the reactivity of an already sensitive adolescent reward system, could diminish the still limited capacity of a comparatively immature cognitive control system, or both. To date, a limited body of evidence seems to favor the former explanation—that the presence of peers increases adolescent risk-taking by increasing reward sensitivity (Chein et al., 2011; O’Brien, Albert, Chein, & Steinberg, 2011; Segalowitz et al., 2012; Smith, Steinberg, Strang, & Chein, 2015; Weigard, Chein, Albert, Smith, & Steinberg, 2013). For example, one study (Chein et al., 2011) found that adolescents’ increased risk taking in the presence of peers was paired with heightened engagement of the ventral striatum and orbitofrontal cortex (OFC), regions thought to be critically involved in the valuation and experience of reward. Meanwhile, though adults demonstrated greater activation of control regions (lPFC) during decision making than did adolescents, social context had no effect on control circuitry in either age group, suggesting that peers influence adolescent risk taking by modulating reward, but not control, processes.
An important caveat, however, is that performance on the task used in the Chein et al. (2011) study, a driving simulation game referred to as the stoplight game, appears to be driven more by sensation seeking than impulse control (Chein et al., 2011; Steinberg et al., 2008), suggesting that this task may be more sensitive to the influence of peers on reward processes than on cognitive control processes. In order to observe peer influences on the behavioral and neural correlates of cognitive control, it may be necessary to utilize a task that draws more directly upon cognitive control mechanisms.
In the current study, we explored the peer effect on adolescent decision making during two tasks with interleaved rounds of play, one emphasizing risk taking, and one emphasizing cognitive control. Adolescents, ages 15 to 17 years, performed a probabilistic gambling task (risk taking) and a go/no-go response-inhibition task (cognitive control) while undergoing functional magnetic resonance imaging (fMRI). To examine social context effects, the tasks were completed either alone or under the belief that an anonymous peer was watching. In line with past studies, we hypothesized that adolescents who completed the risk-taking task while believing that a peer was observing them would make more risky decisions than adolescents who completed the task alone. Furthermore, we predicted that this behavioral effect would be paired with increased engagement of reward circuitry, and specifically the striatum and OFC. Conversely, and in accord with the Chein et al. (2011) findings, we hypothesized that peer observation would not significantly affect behavior or engagement of control regions, such as the lPFC, during the response-inhibition task.
Method
Participants and recruitment
Twenty-eight adolescent participants (ages 15–17 years, M = 16.3, SD = 0.74, 19 females) were included in the current analysis. We focused on 15–17-year-olds because this is thought to be a peak period of risk propensity, during which reward and social sensitivity are elevated and novel opportunities to engage in risky behaviors arise (for review, see Shulman et al., 2016). These individuals were a subsample of participants who had successfully completed both the risk-taking and response-inhibition tasks in the scanner, as part of a larger project. Behavioral results from the probabilistic gambling task, based on a partially overlapping sample of participants, have been previously reported (Smith et al., 2014a).
Prior to participation, all adolescents provided assent and parental consent, and were evaluated for MRI contraindications. Participants were randomly assigned to complete the tasks either unobserved (the “alone” condition, N = 14, nine females) or under the deception that a same-aged, same-sex peer was observing their performance from a neighboring room (the “peer” condition, N = 14, six females). The two groups did not differ in age, t(26) = .80, p = .43, gender, χ2(1, N = 28) =1.29, p = .26, race, χ2(2, N = 28) = 3.85, p = .15, or ethnicity, χ2(2, N = 28) =.38, p = .83.
All participants underwent MRI scans at Temple University Hospital’s Magnetic Resonance Imaging Center (Philadelphia, PA). All protocols and procedures were approved by the university’s institutional review board.
Procedure
While in the scanner, participants completed three rounds of a probabilistic gambling task (PGT) and two rounds of a go/no-go (GNG) task, a commonly used response-inhibition task. Importantly, the tasks were completed in an interleaved fashion, beginning with PGT and alternating between the two tasks (the task sequence was always PGT-GNG-PGT-GNG-PGT). By interleaving the tasks within individuals, and within a single social context (alone or peer), we could ensure that the peer observation manipulation would be equally salient across the two paradigms. Prior to the scanning session, participants were informed that their performance on both tasks would determine the proportion of a $15 “bonus” that they would receive upon study completion. All participants, regardless of task behavior, received the full $15 bonus once they completed both tasks.
Probabilistic gambling task
On each trial of the PGT, participants were presented with a wheel that was divided into three distinct pie-shaped sections, each colored green, red, or gray (see Fig. 1a). Participants were informed that if they chose to “play” the wheel, it would spin and land on one of the three sections. The green section indicated the opportunity to win tokens (reward, +10 tokens), red indicated the opportunity to lose tokens (loss, −10 tokens), and gray indicated the chance of neither winning nor losing tokens (neutral outcome, 0 tokens). It was explained that the relative size of each section was exactly indicative of the chance of landing on that section and achieving the specified outcome.
a. Probabilistic gambling task. Participants decided whether to play or pass on the wheel on the basis of the gain-to-loss probability ratio presented by the colored sections of the wheel. Risk-taking was measured by the percentage of plays. b. Gain-to-loss probability ratios ranged from safe (low probability of loss) to risky (high probability of loss). c. Go/no-go task (modified from Casey et al., 2007). Participants pressed a button as quickly as possible when presented with letters (go trials) but withheld the button press when presented with the letter “X” (no-go trials). (Color figure online)
Participants each began the task with a “bank” of 100 tokens. Token values were not equated to any specific monetary value. On each trial, participants were shown a wheel and asked to think for 2 seconds about whether they would like to “play” (and accept the outcome of the wheel’s spin) or “pass” (move on to the next wheel). Next, the words “Play or Pass?” appeared above the wheel, and participants were required to make their selection within 1,500 ms. At the beginning of each round, participants were told that the outcome for the round was completely independent of prior rounds.
If a participant chose to play on a wheel, the wheel began spinning and came to rest with an indicator pointing to one of the three sections. If the participant chose to pass, a screen indicating “no play” appeared. After each trial, a feedback screen displayed the trial’s outcome (+10 for a win, −10 for a loss, and 0 for a neutral or pass) and the updated overall game earnings (see Fig. 1a). All participants completed an initial instructional session with the researcher, followed by a practice session consisting of 10 wheels. Participants played three, 8-minute rounds of 42 wheels each.
Six different gain-to-loss probability ratios, ranging from 1.5 to 0.33, were used (see Fig. 1b). The neutral portion of the wheel was always fixed at either 50% or 10%, and the variably sized gain and loss sections completed the wheel. The rewarding portion of the wheel (i.e., green section) was alternated between being presented on the right or left side of the wheel in a randomized order. Risk-taking was measured by the percentage of plays (as opposed to passes) on each wheel configuration.
Previous analyses of the effects of peer presence on PGT performance revealed that social context differentially influenced behavior as a function of the risk-to-reward ratio of the wheel, leading us to consider two distinct wheel categories, relatively safe and relatively risky (Smith et al., 2014b). Peers did not increase overall decisions to play on “safe” wheels, in which the likelihood of loss was low (gain–loss probabilities: 1.5, 1.0, 0.81). However, the presence of peers led to significantly greater risk-taking on “risky” wheels, where the likelihood of loss was high (gain–loss probabilities: 0.67, 0.5, 0.33). Accordingly, several of the behavioral and neuroimaging analyses in the current manuscript treat these wheel categories separately.
Go/no-go task
The GNG task used in the current study was modified from Casey et al. (2007). In this task, letters were presented successively for 500 ms, separated by a 2,000-ms intertrial interval. Once a letter appeared, participants were required to press a button as quickly as possible (go trials), except when the presented letter was an “X.” Participants were instructed to withhold the button press when an X appeared (no-go trials; see Fig. 1c). Twenty percent of the overall trials were no-go trials. Four possible behaviors could occur in this task. In a go trial, a participant could (1) correctly respond (“hit”) or (2) fail to respond (“miss”); in a no-go trial, a participant could (3) correctly withhold a response (“correct rejection”), or (4) fail to withhold a response (“false alarm”). Participants completed two runs of the task, each lasting about 5 minutes. Each run included 128 trials (103 go trials, 25 no-go trials). Due to a timing glitch with the computer running the task, some trials were excluded because the intertrial interval was extremely short (less than 10 ms), not allowing enough time for the participant to respond. After excluding these short trials, there were an average of 104.43 ± 22.42 go trials and 25.75 ± 7.25 no-go trials per participant across groups. Importantly the number of available go, t(26) = −.16, p = .88, and no-go, t(26) = .33, p = .74, trials did not differ by social context group (i.e., peer vs. alone).
Social context manipulation
Participants were randomly assigned to complete the study test battery either alone (N = 14) or under the belief that their performance was being observed by a peer (N = 14). Those in the “peer” condition were led to believe that another adolescent participant of the same gender was seated in a different room in the building and would be observing their performance on a battery of computer tasks via a closed-circuit computer system. Participants were told that the observer would be making predictions about task performance, and in order to inform those predictions, the observer and the participant would engage in a brief introductory exchange over an intercom. In reality, there was no observer, and the observer’s introduction was a prerecorded audio file. During the exchange, the following voice recording was played: “Hey, my name is John (“Jess,” for female participants), I’m [participant’s age] years old. My favorite color is blue and I was born in Philadelphia.” It was explained that a purpose of the study was simply to see if the observer could make accurate predictions about the player’s performance without physically meeting the observed participant. Participants were also told that they would briefly meet the observer at the end of the study.
Following the social exchange, participants began the tasks. To remind the participant of the peer observer (and to therefore keep the social manipulation salient), just prior to each round of play, the experimenter asked, over the intercom, if the participant was ready to begin the task, and subsequently asked the observer if he or she was finished making predictions and ready for the participant to begin. The observer’s prerecorded response, played over the intercom, was a simple confirmation each time: (e.g., “OK, I’m ready now,” “I’m good, go ahead”). To establish that only the anonymous peer was observing the participant (i.e., that the experimenter was not observing the participant) the experimenter informed the participant that he or she would be located in a separate area next to the testing room and did not have access to the closed circuit viewing system.
In the “alone” condition, participants completed the same tasks without any reference to an observer. As in the peer condition, participants in the alone condition were told that the experimenter would not be able to observe the participant’s performance. Participants were debriefed via a mailed letter following completion of the study to ensure no communication of the manipulation among participants.
fMRI data acquisition
Participants were scanned using a 3-Tesla Siemens magnet, equipped with a 12-channel phased array transmit/receive head coil. A T-1 weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) image, collected in the sagittal plane, provided high-resolution structural images for coregistration of functional images and inter-participant normalization. Each functional scan of the task involved a whole brain T2*-weighted echoplanar imaging (EPI) sequence (TR = 2,000 ms, TE = 30 ms, flip = 90°, 4-mm slice thickness with no gap, 220 × 220 field of view, 3.4 × 3.4-mm in-plane resolution).
fMRI data analysis
All fMRI data analyses were performed using AFNI (Cox, 1996). The functional data were preprocessed in several steps. First, data were interpolated to adjust for slice time acquisition effects. Next, a six-parameter rigid-body motion correction was applied and the motion-corrected functional and structural images were coregistered. All participants included in the analysis exhibited less than 2-mm of motion and less than 2 degrees of rotation in any direction/axis over the course of the scan. Motion-corrected images were smoothed with a 6-mm full-width half maximum Gaussian kernel before applying a probabilistic mask to exclude voxels located in white matter, ventricles, or outside of the brain. Data were then converted based on voxel-wise percent signal change (relative to the mean value for each voxel across the run), and finally, all functional scans were normalized into MNI space. The preprocessed data from each participant were analyzed in an event-related fashion using a general linear model (GLM) approach. All PGT analyses focused on the decision-making period of the task, and were time-locked to the button press that signaled the decision to either play or pass. GNG analyses used events time-locked to the presentation of each successive stimulus. Events for each task were convolved with a canonical model of the hemodynamic response function (Boynton, Engel, Glover, & Heeger, 1996).
All whole-brain group analyses were performed using individual participants’ voxel-wise parameter estimates (beta coefficients) from each GLM. The group maps were corrected for multiple comparisons using a voxel-wise probability threshold (p < .001) and a contiguity requirement (43 adjacent voxels), resulting in a family-wise error rate of p = .05, as determined by 10,000 Monte Carlo simulations. To estimate spatial smoothness, we utilized the spatial autocorrelation function provided in AFNI (for both 3dFWHM and 3dClustSim; Cox, Chen, Glen, Reynolds, & Taylor, 2017), which gave estimates of a = 0.361, b = 10.040, c = 7.719. All results are reported in MNI coordinates.
Region-based analysis
Across both risk-taking and reward processing studies, the peak coordinates of developmental and social context effects can vary. For instance, Chein et al., (2011) identified an Age × Social Context interaction in the ventral portion of the striatum. Meanwhile, using a gambling task, Braams, Peters, Peper, Güroğlu, and Crone (2014) found heightened activation for more dorsal regions of the striatum (caudate) on trials where participants gambled to receive a reward for themselves, compared to when they played for a friend or antagonist. To address this variability in activation sites, for each task, we extracted regions-of-interest based on an unbiased (with respect to social context) whole-brain GLM analysis that identified the loci of general task-dependent activation, and then selected regions of a priori interest occurring within the resulting group maps for further region-specific analysis.
Research has highlighted the involvement of the striatum and OFC during adolescent reward processing and risk taking (e.g., Braams et al., 2014; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Galván et al., 2006; Van Leijenhorst et al., 2010), and shown that this activation is strengthened in the presence of peers (Chein et al., 2011). Accordingly, we anticipated that, during the PGT, we would observe overall activation within these regions in a GLM contrast of decisions to play versus decisions to pass (collapsing across all wheel categories and social contexts). The full GLM model contrasting play versus pass decisions (feedback was not included in the model) also included nuisance covariates representing each of the six estimated motion time series provided by motion correction as well as covariates representing linear and higher order drift trends. This overall task contrast failed to reveal any task-specific activation within the OFC at the thresholded group level, but did indicate bilateral activation of the striatum—localized specifically within the caudate (with the cluster extending into adjacent regions of the anterior insula and thalamus). (See Fig. 2a and Table 1 for a full list of regions from this contrast.) To examine social-context and wheel-category effects, we extracted data from a 6-mm sphere centered at the peak coordinates of the caudate activation site (see Fig. 2b), and submitted the average percent signal data from those regions to additional analysis in SPSS.
Social context effects during risky decision making. a. Compared to the alone group, participants in the peer group exhibited higher activation of several regions, including the striatum, during risk taking (pictured in orange, threshold p < .001, 33 voxel extent). b. 6-mm mask of peak coordinates for region-based analyses. (Color figure online)
We took a similar approach in order to identify brain regions activated in association with the GNG task, a task that typically engages response inhibition regions of the lateral and medial prefrontal cortex (for review see, Criaud & Boulinguez, 2013). In the GLM for this task, each event of interest (correct rejections, false alarms, and hitsFootnote 1) was included in the model, with events time-locked to stimulus onset. Nuisance covariates representing each of the six estimated motion time series, drift trends, and trials that were excluded due to timing errors (described above) were also included as regressors in the model. While some GNG studies examine activation on all go trials compared to all no-go trials, ignoring accuracy (Criaud & Boulinguez, 2013), others examine only correct trials (e.g., Casey et al., 2007). We chose to align our analyses with the latter approach for two reasons. First, we were interested in whether social context affected brain activation when participants were successfully engaging cognitive control (as will be discussed in more detail below). Second, while it may be interesting to explore whether peers influence cognitive control circuitry when teens fail to correctly inhibit a response (i.e., in false-alarm trials), participants committed very few false alarms overall (M = 5.96, SD = 3.54), which left insufficient power to examine this effect neurally (in contrast, the mean number of correct rejections per participant was 19.80, SD = 5.94). As in the PGT analysis, GNG regions of interest were created using a 6-mm sphere around the peak activations observed during response inhibition (correct rejections compared to hits, collapsed across social context), which included several regions commonly associated with response inhibition: bilateral IFG/insula, the anterior cingulate, and the middle frontal gyrus (see Fig. 3 and Table 2 for a full list of regions).
Other regions and whole-brain analysis
For completeness, we conducted three further analyses. First, while region-of-interest analyses focused on areas of a priori interest that emerged in association with general task contrasts, task activation was also observed in a few other regions. Accordingly, we explored possible social context effects in each of the remaining regions showing task-related activation during the risk-taking and response inhibition tasks, using the same approach we used to test for these effects in a priori regions of interest. In addition, we conducted whole-brain analyses examining social context effects on a voxel-wise basis for both the PGT and GNG tasks. For PGT, a second GLM was also performed to examine social context effects (peer vs. alone) during decisions to play separated by wheel category (Smith et al., 2014a). In the GNG task, we examined the effects of social context (peer vs. alone) on hits compared to correct rejections.
Finally, we explored sensitivity to the social context manipulation in regions that were mutually activated by the two tasks. First, a simple conjunction (intersection) analysis was conducted to find regions that were activated in common across the two tasks (GNG contrast of CR > Hits, PGT contrast of Plays > Passes). This analysis revealed clusters in the right (126 voxels, 38, 24, −3) and left anterior insula (93 voxels, −28, 21, −4) as the only loci of intersection (see Fig. 4). Follow-up analyses treated these regions of overlap as ROI’s, and assessed whether activation exhibited main or interactive effects of task (risky plays, correct rejections) and social context (peers, alone). These analyses allowed us to test whether participants exhibiting relatively greater activation during risk taking also evinced relatively weaker activation during control, and importantly, to explore whether this relationship varied as a function of social context.
Results
Task behavior
Probabilistic gambling task
Participants in both social conditions behaved in a systematic fashion, playing proportionately more often when presented with the safe wheels (higher gain-to-loss probabilities) and less often when presented with risky wheels (when the gain-to-loss probability was lower; safe: M = 64.28%, SD = 15.95; risky: M = 15.76%, SD = 16.02).
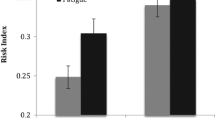
A 2 × 2 (Social Context × Wheel Category) repeated-measures ANOVA was conducted, with social context (alone, peer) as a between-subjects variable and wheel category (safe, risky) as a within-subjects variable. As seen in Smith et al. (2014a) (which included a larger sample of participants who did not also complete the GNG and thus were excluded from the present report), there was a significant main effect of wheel type on decision-making, F(1, 26)=265.70, p< .001; all participants played more often on safe than on risky wheels. There was also a main effect of social context on decision-making, F(1, 26) = 6.83, p = .02; adolescents in the peer condition played more often than adolescents who completed the task alone. The interaction between social context and wheel type did not reach significance, F(5, 130) = 1.27, p = .28, but followed a trend across gain–loss probabilities that we had previously observed with this task. Namely, as seen in the larger sample (Smith et al., 2014a), there were no differences in the percentage of wheels played in the peer and alone conditions for the most favorable wheel types, safe: t(26) = 1.55, p = .13 d = 0.61, but participants in the peer condition, compared to the alone condition, showed a significantly higher percentage of plays on the riskier wheel types, risky: t(26) = 2.95, p = .007, d = 1.16, an effect that survives Bonferroni-correction for multiple testing across wheel types; see Fig. 5).
In addition, individuals in the peer group earned significantly more points than individuals in the alone group, t(26) = 2.56, p = .02, d = 1.00 (alone: M = 128.57, SD = 15.11; peer: M = 145.00, SD = 18.71).
Go/no-go task
Overall, participants successfully inhibited their responses on an average of 77.37% of no-go trials, with success on no-go trials ranging from 47.06% to 92.31% across individuals. To test for any possible social context effects on response inhibition, we ran an independent-samples t test on the percentage of correct rejections, but found no group differences, t(26) = 0.20, p = .84, d = 0.08 (alone group: M = 76.97%, SD = 12.61; peer group: M = 77.76%, SD = 7.74%; see Fig. 6). To test the possibility that social context affected participants’ reaction time (RT), but not accuracy, we ran additional t tests examining group differences in RT on hits and false alarms. There were again no differences between participants’ RTs in the alone and peer conditions on hits, t(26) = 0.68, p = .50, d = 0.27 (alone group: M = 330.96, SD = 39.93; peer group: M = 340.58, SD = 34.46), or false alarms, t(26) = 0.07, p = .94, d = 0.03 (alone group: M = 368.04, SD = 28.90; peer group: M = 367.33, SD = 21.64).
fMRI results
PGT: Region-based analysis of social context effects on risk-taking
Using the caudate region identified in Table 1, we first ran mixed-effects ANOVAs to examine the impacts of social context (peer vs. alone) and wheel category (risky vs. safe) on activation during risk-taking (decisions to play). As predicted, the caudate exhibited a main effect of social context, F(1, 26) = 4.74, p = .04, ηp2 = .15, wherein those in the peer condition exhibited significantly greater activation than those who completed the task alone during decisions to play. There was also a significant main effect of wheel category on caudate activation, F(1, 26) = 24.80, p < .001, ηp2 = .48, indicating greater activation during decisions to play on safe than on risky wheels. Although there was no overall interaction between social context and wheel category, follow-up tests demonstrated that the effect of social context was specific to decisions to play on risky wheels, t(26) = 2.84, p = .009, d = 1.11, whereas there were no group differences during decisions to play on safe wheels, t(26) = 0.68, p = .50, d = 0.27.
Additional post-hoc contrasts shed further light on the factors that drive caudate activation and its sensitivity to social context. Specifically, those in the alone condition exhibited no change in caudate activation for a contrast of activation for all decisions to play (i.e., pursue the reward) versus all decisions to pass, t(13) = 1.526, p = .151)—that is, gambling on the offer did not in itself increase caudate engagement. By comparison, the same contrast in the peer group was highly significant, t(13) = 3.043, p = .009. Finally, no social-context effects are observed in the caudate when we collapse across all trial types (safe and risky choices, decisions to play and pass), t(26) = 1.41, p =.17, presumably because being observed by peers does not increase caudate activation when the “safe” choice is selected, and so the overall peer effect is diluted. Put together, the data suggest that the peer context amplifies caudate signaling selectively when a gamble is pursued despite poor odds, such that it was the combination of the risky gamble and peer observation that evinces evidence of a social context effect.
To test whether stronger engagement of the caudate was indeed associated with greater risk-taking, a further correlation was run between the percent signal change observed in the caudate (averaged across hemispheres) for a given individual and the percentage of that individual’s plays on risky wheels. The correlation revealed that activation of the striatum was strongly positively correlated with an individual’s overall risk-taking behavior (r = .57, p = .002; see Fig. 7).
GNG: Region-based analysis of social context effects on inhibition
We examined the impact of social context (peer vs. alone) on regions engaged in association with GNG inhibitory control. There were no significant main effects of social context on activation in the right, F(1, 26) = 0.54, p = .47, ηp2 = .02) or left, F(1, 26) = 0.16, p = .90, ηp2 = .001) IFG. There was, however, a significant main effect of social context on activation in a small region within the right posterior middle frontal gyrus, F(1, 26) = 6.53, p = .02, ηp2 =.20, with this region exhibiting significantly more activity among those in the alone condition than in the peer condition. As we did with respect to caudate activation during the PGT, we tested the GNG right posterior middle frontal activation for an overall correlation with individual differences in successful inhibition rate, but this correlation failed to reach significance (r = −.25, p = .19).
Other regions and whole-brain analyses
As noted, an intersection analysis revealed overlapping activation in the anterior insula, bilaterally, for the two task contrast maps (Fig. 4). ROI analysis based on activation patterns within these areas of overlap explored potential social context effects within and across tasks. A repeated-measures ANOVA revealed a significant interaction in the right, but not left, insula, right: F(1, 26 4 .29, p < .05, ηp2 = 0.14; left: F(1, 26) = 2.19, p = ns, ηp2 = 0.08. To decompose the interaction, we ran independent-sample t tests testing for significant social context group differences during the PGT (plays on risky wheels) and GNG (correct rejections), separately. These analyses showed significant group differences during risky plays, left: t(26) = 2.82, p = .009; right: t(26) = 3.51, p = .002, but not during correct rejections, left: t(26) = 0.30, p = ns; right: t(26) = 0.56, p = ns. More specifically, in PGT, those in the peer group showed greater anterior insula activation during decisions to play on risky pies compared to those in the alone group. Further paired-sample t -tests revealed significant activation differences in the right, t(13) = 3.05, p = .009, but not the left, t(13) = 1.33, p = ns, insula when comparing activation for the PGT versus GNG tasks in the peer group. There was not a significant difference in insula activation in the alone group, left: t(13) = 0.74, p = ns; right: t(13) = 0.46, p = ns. Together, these follow-up tests indicate that the significant interaction we observed is driven by higher activation in the peer group during decisions to play on risky wheels.
No other region activated in association with the PGT or GNG task contrasts (see Tables 1 and 2) was significantly impacted by the manipulation of social context. Voxel-wise whole-brain analyses examining social-context effects for each task also failed to produce any further region showing a significant association with the social-context manipulation, after correction for multiple testing.
Discussion
The current study shows that the manipulation of social context impacts behavior in a risk-taking task as well as activation of the striatum and anterior insula during risky decision making. In contrast, there was no impact of social context on response-inhibition behavior, and the only observed effect on brain activation during the GNG task occurred within a circumscribed region of the posterior middle frontal gyrus that is not typically engaged in association with response inhibition.
These findings add to a growing literature demonstrating the impact of peer presence on adolescent risk taking and reward sensitivity (Gardner & Steinberg, 2005; O’Brien et al., 2011; Weigard et al., 2013) and on the engagement of reward-processing regions (Chein et al., 2011; Smith et al., 2015). Of note, the exact subregion of the striatum where social-context effects emerge may be contingent on the task and/or analysis employed (e.g., Cascio et al., 2014; Chein et al., 2011; Smith et al., 2015). Whereas both dorsal and ventral subregions of the striatum are implicated in the experience and valuation of rewards, the specific locus of activation observed when adolescents are performing tasks in the presence of peers may have important implications for our understanding of the peer effect. For example, the neural peer effect might be limited to the caudate for tasks that involve evaluation of explicitly described reward outcomes, such as the PGT used in the present study and a high/low card guessing task we have previously employed in our lab (Smith et al., 2015). Alternatively, in tasks such as the stoplight task (Chein et al., 2011), where outcomes of risk (e.g., the likelihood of crashing at a given light) are not explicitly described and must be learned through task experience, the neural peer effect appears to localize in more ventral regions of the striatum. In the present study, caudate activation was overall greatest during decisions to play on safe wheels, for which the potential for reward is particularly high. The fact that this pattern was present for both the alone and peer groups suggests that this caudate activity might reflect a reward prediction or anticipation signal. Although this region was found to be generally less strongly engaged when the odds of a reward were lower (i.e., when a risky offer was presented), peer observation concomitantly increased participants’ tendencies to pursue these less favorable offers and the degree to which the caudate was active during this decision phase. Taken together, we interpret this result to indicate that peers heighten the salience of anticipated rewards.
Another region that emerged as a potential target during our analysis of the data was the anterior insula. We have previously suggested that the anterior insula is a pivotal region during adolescence, and more specifically during cognitive–emotional interactions (Smith, Steinberg, & Chein, 2014b). In particular, as this region continues to develop its role as a cognitive–-emotional hub (Menon & Uddin, 2010), adolescents are particularly susceptible to emotional arousal, such as the presence of peers. This arousal is more readily communicated to reward-processing regions, such as the striatum, than to self-regulatory regions, such as the dorsolateral prefrontal cortex, in turn increasing sensation-seeking behaviors (see Smith et al., 2014b). The absence of social context effects in anterior insula activation during GNG may imply that the “cold” control task needs an affective task component (i.e., faces, monetary reward, feedback), in addition to social arousal, to influence behavior. Studies examining the role of the anterior insula on control and reward processes are needed to further this theory.
Although the impact of peers on adolescent risk-taking is usually described in a negative light, it is important to note that individuals in the peer condition in the present study actually succeeded in accumulating more “points” on the PGT task—and hence were more rewarded—than those who completed the task alone. As such, rather than being maladaptive, as is typically assumed in discussions of adolescent risk behavior, the peer influence on risk task behavior observed in the present study resulted in overall more favorable outcomes. Such findings raise the interesting possibility of using positive peer influence to evoke desirable behaviors, such as by stimulating “risky” actions in the classroom (e.g., speaking up to answer questions or working with a new classmate). Indeed, in other studies, we have shown that the presence of peers facilitates learning among late adolescents by increasing sensitivity to both positive and negative feedback (Silva, Shulman, Chein, & Steinberg, 2015).
As discussed above, based on dual systems models it might be expected that an increase in reward sensitivity during peer observation could, through interactions taking place between the reward and control circuitry, also make adolescents less effective at engaging cognitive control. The present study provides mixed evidence with respect to this hypothesis. On the one hand, we found no evidence that social context affects response-inhibition behavior, with no effect on either accuracy or response times. However, we did find that adolescents in the peer condition, relative to those tested alone, demonstrated diminished activation of the right posterior middle frontal gyrus. This diminished middle frontal gyrus engagement in the peer context could be interpreted as a reflection of disrupted, and hence less efficient, control processing during inhibition (although see Poldrack, 2015, for a discussion of the inadequacy of the “efficiency” account). However, this prefrontal region is somewhat posterior to the mid-frontal region most typically implicated in inhibitory control. Furthermore, across participants, activation of this region was not strongly associated with task performance (successful inhibition), suggesting that the group difference in activation was not directly relevant to task behavior.
The current study has several limitations that should be acknowledged and perhaps addressed in future research. First, the sample size for each group was small. While we tried to be thorough in exploring all patterns in the data, including null effects, it is possible that some of the analyses were underpowered. These findings need to be replicated in a larger sample to ensure that our results and conclusions are warranted. Second, the response inhibition task turned out to be very easy for some participants and accordingly did not produce a high rate of false alarms in those subjects. As a result, analysis of regional activation was limited to contrasts involving successful inhibition (correct rejections) rather than failed inhibition (false alarms). It is possible that a more difficult measure of cognitive control, or one that required control over emotional or affectively evocative stimuli, would yield more substantial social context effects (see Botdorf, Rosenbaum, Patrianakos, Steinberg, & Chein, 2017). Finally, the present study uses two interleaved tasks rather than one task that explores social-context effects on both reward processing and self-regulation. While we examined the data for possible order effects and differences in task behavior across runs, a stronger design is needed for future studies. In addition to task design (i.e., measuring both constructs within one task), having all participants complete the task alone and in the presence of peers would strengthen our ability to interpret the social-context findings.
In conclusion, our findings extend the current literature by demonstrating that the presence of peers affects adolescents’ risk behavior even when it does not affect their ability to inhibit a response during a non-affective cognitive-control task. Together, these findings support the conclusion that the presence of peers biases adolescent risky decision making by increasing reward sensitivity, and more specifically, by affecting the recruitment of reward-processing regions, rather than by diminishing adolescents’ capacity to engage cognitive control.
Notes
To ensure that the model was not overdetermined, we excluded misses from the regression.
References
Albert, D., Chein, J., & Steinberg, L. (2013). The teenage brain: Peer influences on adolescent decision making. Current Directions in Psychological Science, 22(2), 114–120. https://doi.org/10.1177/0963721412471347
Botdorf, M., Rosenbaum, G. M., Patrianakos, J., Steinberg, L., & Chein, J. M. (2017). Adolescent risk-taking is predicted by individual differences in cognitive control over emotional, but not non-emotional, response conflict. Cognition and Emotion, 31(5), 972–979.
Boynton, G. M., Engel, S. A., Glover, G. H., & Heeger, D. J. (1996). Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience, 16(13), 4207–4221.
Braams, B. R., Peters, S., Peper, J. S., Güroğlu, B., & Crone, E. A. (2014). Gambling for self, friends, and antagonists: Differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage, 100, 281–289.
Cascio, C. N., Carp, J., O’Donnell, M. B., Tinney, F. J., Jr, Bingham, C. R., Shope, J. T., & Falk, E. B. (2014). Buffering social influence: neural correlates of response inhibition predict driving safety in the presence of a peer. Journal of Cognitive Neuroscience, 27(1), 83–95.
Casey, B. J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319.
Casey, B. J., Getz, S., & Galván, A. (2008). The adolescent brain. Developmental Review, 28(1), 62–77. https://doi.org/10.1016/j.dr.2007.08.003
Casey, B. J., Epstein, J., Buhle, J., Liston, C., Davidson, M. C., Tonev, S., . . . Glover, G. (2007). Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry, 164, 1729–1736.
Chein, J., Albert, D., O’Brien, L., Uckert, K., & Steinberg, L. (2011). Peers increase adolescent risk-taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–F10. https://doi.org/10.1111/j.1467-7687.2010.01035.x
Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173.
Cox, R. W., Chen, G., Glen, D. R., Reynolds, R. C., Taylor, P. A. (2017). FMRI clustering in AFNI: false-positive rates redux. Brain Connectivity, 7(3), 152–171
Criaud, M., & Boulinguez, P. (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience and Biobehavioral Reviews, 37, 11–23.
Crone, E. A., & Dahl, R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650.
Ernst, M. (2015). The triadic model perspective for the study of adolescent motivated behavior. Brain and Cognition, 89, 104–111.
Eshel, N., Nelson, E. E., Blair, R. J., Pine, D. S., & Ernst, M. (2007). Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia, 45(6), 1270–1279.
Galván, A. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience. https://doi.org/10.3389/neuro.09.006.2010
Galván, A., Hare, T. A., Parra, C. E., Penn, J., Voss, H., Glover, G., & Casey, B. J. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience, 26(25), 6885–6892.
Gardner, M., & Steinberg, L. (2005). Peer influence on risk-taking, risk preference, and risky decision-making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. https://doi.org/10.1037/0012-1649.41.4.625
Luciana, M., & Collins, P. F. (2012). Incentive motivation, cognitive control, and the adolescent brain: Is it time for a paradigm shift?. Child Development Perspectives, 6(4), 392-399.
Luna, B., & Wright, C. (2016). Adolescent brain development: Implications to the juvenile criminal justice system, In K. Heilbrun (Ed.), APA Handbook of Psychology and Juvenile Justice, Washington, DC: American Psychological Association.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214, 655–667.
O’Brien, L., Albert, D., Chein, J., & Steinberg, L. (2011). Adolescents prefer more immediate rewards when in the presence of their peers. Journal of Research on Adolescence, 21(4), 747–753. https://doi.org/10.1111/j.1532-7795.2011.00738.x
Pfeifer, J. H., & Allen, N. B. (2012). Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences, 16, 322–329.
Poldrack, R. A. (2015). Is “efficiency” a useful concept in cognitive neuroscience? Accident Analysis and Prevention, 11, 12–17. https://doi.org/10.1016/j.dcn.2014.06.001
Segalowitz, S. J., Santesso, D. L., Willoughby, T., Reker, D. L., Campbell, K., Chalmers, H., & Rose-Krasnor, L. (2012). Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Social Cognitive and Affective Neuroscience, 7(1), 115–124. https://doi.org/10.1093/scan/nsq090
Shulman, E. P., Smith, A. R., Silva, K., Icenogle, G., Duell, N., Chein, J., & Steinberg, L. (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17(2015), 103–117. https://doi.org/10.1016/j.dcn.2015.12.010
Silva, K., Shulman, E., Chein, J., & Steinberg, L. (2015). Peers increase late adolescents’ exploratory behavior and sensitivity to positive and negative feedback. Journal of Research on Adolescence. Advance online publication. https://doi.org/10.1111/jora.12219
Smith, A. R., Chein, J., & Steinberg, L. (2013). Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Hormones and Behavior, 64(2), 323–332. https://doi.org/10.1016/j.yhbeh.2013.03.006
Smith, A. R., Chein, J., & Steinberg, L. (2014a). Peers increase adolescent risk-taking even when the probabilities of negative outcomes are known. Developmental Psychology, 50(5), 1564–1568. https://doi.org/10.1037/a0035696
Smith, A.R., Steinberg, L. & Chein, J. (2014b). The role of the anterior insular cortex in adolescent decision-making. Developmental Neuroscience, 36, 196–209.
Smith, A. R., Steinberg, L., Strang, N., & Chein, J. (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience, 11, 75–82. https://doi.org/10.1016/j.dcn.2014.08.010
Steinberg, L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. https://doi.org/10.1016/j.dr.2007.08.002
Steinberg, L. (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52, 216–224. https://doi.org/10.1002/dev.20445
Steinberg, L., Albert, D., Cauffman, E., Banich, M., Graham, S., & Woolard, J. (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 44(6), 1764–78. https://doi.org/10.1037/a0012955
Van Leijenhorst, L., Moor, B. G., de Macks, Z. A. O., Rombouts, S. A., Westenberg, P. M., & Crone, E. A. (2010). Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage, 51(1), 345–355.
Weigard, A., Chein, J., Albert, D., Smith, A., & Steinberg, L. (2013). Effects of anonymous peer observation on adolescents’ preference for immediate rewards. Developmental Science, 17(1), 71–78. 10.1111/desc.12099
Zuckerman, M. (1994). Behavioral expressions and biosocial bases of sensation seeking. New York, NY: Cambridge University Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, A.R., Rosenbaum, G.M., Botdorf, M.A. et al. Peers influence adolescent reward processing, but not response inhibition. Cogn Affect Behav Neurosci 18, 284–295 (2018). https://doi.org/10.3758/s13415-018-0569-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0569-5