Abstract
Recent interest has emerged in understanding the neural mechanisms by which deficits in emotion regulation (ER) early in development may relate to later depression. Corticolimbic alterations reported in emotion dysregulation and depression may be one possible link. We examined the relationships between emotion dysregulation in school age, corticolimbic resting-state functional connectivity (rs-FC) in preadolescence, and depressive symptoms in adolescence. Participants were 143 children from a longitudinal preschool onset depression study who completed the Children Sadness Management Scale (CSMS; measuring ER), Child Depression Inventory (CDI-C; measuring depressive symptoms), and two resting-state MRI scans. Rs-FC between four primary regions of interest (ROIs; bilateral dorsolateral prefrontal cortex [dlPFC] and amygdala) and six target ROIs thought to contribute to ER were examined. Findings showed that ER in school age did not predict depressive symptoms in adolescence, but did predict preadolescent increases in dlPFC-insula and dlPFC-ventromedial PFC rs-FC across diagnosis, as well as increased dlPFC-dorsal anterior cingulate cortex (dACC) rs-FC in children with a history of depression. Of these profiles, only dlPFC-dACC rs-FC in preadolescence predicted depressive symptoms in adolescence. However, dlPFC-dACC connectivity did not mediate the relationship between ER in school age and depressive symptoms in adolescence. Despite the absence of a direct relationship between ER and depressive symptoms and no significant rs-FC mediation, the rs-FC profiles predicted by ER are consistent with the hypothesis that emotion dysregulation is associated with abnormalities in top-down control functions. The extent to which these relationships might confer greater risk for later depression, however, remains unclear.
Similar content being viewed by others
Major depressive disorder (MDD) is a leading cause of disability (World Health Organization, 2016). Deficits in emotion regulation (ER) abilities—also referred to as emotion dysregulation—are thought to contribute to the development and maintenance of depression (Berking, Wirtz, Svaldi, & Hofmann, 2014; Joormann & Gotlib, 2010; Joormann & Quinn, 2014; Silk, Steinberg, & Morris, 2003). Understanding the mechanisms by which emotion dysregulation might heighten the risk for depression has been a topic of growing interest (Joormann & Gotlib, 2010; Rive et al., 2013; Silk et al., 2003). Neuroimaging work in the fields of ER and MDD offer important clues into possible neural mechanisms that might link emotion dysregulation and depressive symptoms. Most notable are studies reporting abnormalities in corticolimbic circuitry—specifically in cognitive control (i.e., prefrontal cortices) and emotion processing areas (i.e., limbic structures)—in individuals with emotion dysregulation (Bebko et al., 2015; Frank et al., 2014) and MDD (Rive et al., 2013; Seminowicz et al., 2004). Disturbances in this circuitry may be one potential mechanism mediating emotion dysregulation and risk for depression. There is some cross-sectional evidence to support this hypothesis (Belden, Pagliaccio, Murphy, Luby, & Barch, 2015; Rive et al., 2013), although a longitudinal examination of the relationships between ER, corticolimbic circuitry, and depressive symptoms is needed to test this mediation hypothesis. Starting such an examination early in development is critical to understanding the developmental psychopathology of MDD, particularly in adolescence, when rates of depression increase (Avenevoli, Swendsen, He, Burstein, & Merikangas, 2015; Merikangas et al., 2010; Wiens et al., 2017). As such, the present study had two main objectives: (1) to examine the extent to which ER in school age predicted depressive symptoms in adolescence, and (2) to test whether variations in corticolimbic rs-FC in preadolescence mediated this relationship.
Emotion regulation, depression, and development
The role of emotion regulation in MDD has been studied extensively (see Joorman & Stanton, 2016, for a review). Findings have demonstrated that depressed individuals exhibit impairments in their ability to use effective ER strategies (e.g., reappraisal) and are prone to use ineffective strategies (e.g., rumination) to regulate emotions. Individual differences in ER have been found to predict variations in depressive symptom severity in adults, with lower ER associated with greater depressive symptoms (Berking et al., 2014). These studies, and others (Feng et al., 2009; Garnefski & Kraaij, 2006; Peeters, Nicolson, Berkhof, Delespaul, & deVries, 2003), suggest that emotion dysregulation may play an important role both in the emergence (Berking et al., 2014) and maintenance of depressive symptoms (Joormann & Gotlib, 2010; Koster, De Lissnyder, Derakshan, & De Raedt, 2011).
Given that childhood and adolescent internalizing disorders are characterized by poor emotion regulation (e.g., rumination; Garnefski, Kraaij, & van Etten, 2005; Siener & Kerns, 2012), the question of whether childhood ER abilities predict the emergence of depression later in development is salient. Findings from Feng et al. (2009) provide support for this hypothesis, reporting that sadness dysregulation in school-age girls (5–8 years old) predicted depressive symptoms in preadolescence (9–10 years old), with this relationship moderated by parenting (Feng et al., 2009). However, given that rates of MDD increase markedly in adolescence (Avenevoli et al., 2015; Merikangas et al., 2010; Wiens et al., 2017), an important follow-up question is whether ER at school age continues to predict depressive symptoms later in adolescence. Given the significant overlap in neural substrates reported in ER and MDD (reviewed below), it will also be important to understand the neural mechanisms by which ER may contribute to risk for MDD. In the sections that follow, we will review the neural correlates of ER and depression, with an emphasis on developmental and resting state functional connectivity (rs-FC) studies to inform our understanding of the relationship between ER and MDD.
Neural correlates of emotion regulation
Functional MRI studies examining emotion regulation in healthy adults have identified brain regions involved in cognitive control and emotion processing. These brain structures include prefrontal regions such as the dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), and dorsal ACC (dACC) and limbic regions such as the amygdala (Frank et al., 2014). During ER, cognitive control (i.e., prefrontal) regions typically exhibit increased activation, while emotion processing (i.e., limbic) structures show reduced activation (Buhle et al., 2014; Frank et al., 2014; Ochsner, Silvers, & Buhle, 2012). This pattern of activation has suggested that successful ER relies on increased frontal control over emotionally reactive regions (Frank et al., 2014). Additionally, the ventromedial prefrontal cortex (vmPFC; Schiller & Delgado, 2010; Winecoff et al., 2013) and anterior insula (Menon & Uddin, 2010) are thought to facilitate communication between frontal and subcortical regions (Ochsner et al., 2012). Together, these regions form a distributed circuit through which executive and emotionally salient information are integrated for flexible and adaptive ER (Buhle et al., 2014; Frank et al., 2014; Ochsner et al., 2012). Importantly, core aspects of this circuit have also been identified during ER in children and adolescents (McRae et al., 2012; Pitskel, Bolling, Kaiser, Crowley, & Pelphrey, 2011; Stephanou, Davey, Kerestes, Whittle, & Harrison, 2017).
Research assessing functional connectivity has begun to elucidate the ways in which connectivity among corticolimbic regions contributes to effective ER. Functional connectivity measures the temporal correlation of neural activity between brain regions during task or rest and is thought to reflect coactivation between brain regions (van den Heuvel & Hulshoff Pol, 2010). Positive connectivity (i.e., cross-correlations greater than zero) represents two brain regions that concurrently increase (or decrease) in spontaneous blood-oxygen-level-dependent (BOLD) signal over time, while negative connectivity (i.e., correlations less than zero) indicates that as one brain region increases in BOLD signal, the other decreases in BOLD signal, over time. The magnitude of the correlation represents the strength in connectivity—correlations closer to 1 and −1 represent stronger connectivity or greater coupling over time, whereas correlations closer to zero represent weaker connectivity. Functional connectivity between the PFC and amygdala, in particular, has been reported during ER tasks (Banks, Eddy, Angstadt, Nathan, & Phan, 2007) and may index information transfer key to emotion regulation. Developmental work suggests that medial PFC–amygdala connectivity during emotion processing changes from childhood into adulthood, such that positive medial PFC–amygdala connectivity shifts to negative connectivity in preadolescence (10 years old; Gee et al., 2013), a trajectory potentially related to improvements in emotion regulation. Functional connectivity at rest (i.e., resting-state functional connectivity; rs-FC) has shown a relationship between PFC–amygdala connectivity and ER success (Uchida et al., 2014), with increasingly negative connectivity associated with greater reppraisal success. Other rs-FC profiles associated with greater ER success include stronger positive rs-FC between the insula-amygdala and vlPFC-amygdala (Morawetz et al., 2016).
Neural correlates of depression
To the extent that corticolimbic connectivity supports healthy ER, disturbances in this circuitry might alter ER. Indeed, rs-FC abnormalities in individuals with emotion dysregulation, including MDD, have been extensively reported. Although some variability in connectivity profiles exist, a recent meta-analysis by Kaiser, Andrews-Hanna, Wager, and Pizzagalli (2015) identified altered rs-FC in several structures comprising the ER corticolimbic network in adults with MDD. Most notably, depressed individuals exhibit (1) decreased connectivity between medial PFC and amygdala—represented as either weaker positive or stronger negative connectivity—and, (2) increased connectivity between dlPFC and medial PFC— represented as stronger positive or weaker negative connectivity (Kaiser et al., 2015). It is worth noting that while these profiles have implications for ER, only one study in this meta-analysis directly examined rs-FC in relation to ER (i.e., rumination; Zhu et al., 2012). More recent studies have reported alterations in PFC-subcortical rs-FC in relation to rumination in depressed adolescents and adults (Lois & Wessa, 2016; Peters, Burkhouse, Feldhaus, Langenecker, & Jacobs, 2016).
Emerging evidence suggests that abnormalities in these corticolimbic structures are also present in children and adolescents with MDD. Abnormalities in medial PFC-amygdala rs-FC has been reported in depressed adolescents, although the direction of rs-FC alterations are mixed. Whereas Connolly et al. (2017) show decreased medial PFC–amygdala connectivity (i.e., weaker positive) in both adolescent boys and girls, Burghy et al., (2013) found increased mPFC–amygdala connectivity (i.e., stronger positive) in depressed adolescent girls. Other rs-FC include decreased (i.e., weaker negative) PFC-dACC (Pannekoek et al., 2014), decreased (i.e., weaker positive) ACC-insula rs-FC (Bebko et al., 2015), and increased (i.e., stronger positive) ACC-amygdala (Chattopadhyay et al., 2017). Rs-FC abnormalities have also been identified in childhood MDD. Work from our group, for example, has shown that early onset depression is associated with atypical connectivity involving the amygdala (Luking et al., 2011), vlPFC (Sylvester et al., 2013), and vmPFC (Gaffrey, Luby, Botteron, Repovš, & Barch, 2012). Further, rs-FC in children at risk for depression show decreased (i.e., stronger negative) dlPFC rs-FC with the ACC and other cognitive control regions (Chai et al., 2016).
Corticolimbic circuit as a mediator
In spite of variability in rs-FC findings, the ER and MDD literatures provide converging evidence that corticolimbic structures supporting ER are compromised in depression. Thus, it is possible that corticolimbic disruptions associated with lower ER might contribute to depressive symptoms. One approach to investigate this hypothesis is to examine whether variations in this circuit mediate the relationship between ER and depressive symptoms. Work showing that individual differences in ER predict variations in medial PFC-amygdala rs-FC (Uchida et al., 2014) offer initial evidence for corticolimbic connectivity as a plausible mediator, although less is known about whether this rs-FC profile predicts depression. Examining this mediation across development will allow a better understanding of the neural trajectories of ER and associated changes in corticolimbic circuitry related to onset of depressive symptoms during the high-risk developmental period of adolescence. Given that mPFC–amygdala connectivity is known to change during preadolescence (Gee et al., 2013), examining rs-FC between these and other corticolimbic structures in preadolescence may reveal important relationships between ER in school age and depression in adolescence.
Prior work from our group examined the cross-sectional relationship between ER and rs-FC in children (Gaffrey et al., 2010; Luking et al., 2011). The present study examined the within-child longitudinal relationships between ER in school age (7–12 years old), rs-FC of the corticolimbic network in preadolescence (9–14 years old), and depressive symptoms in adolescence (10–16 years old) in children with and without a history of MDD. Our first objective was to expand on Feng et al.’s (2009) findings by testing whether (1) ER in school-age girls and boys with and without a history of MDD would predict depressive symptoms in adolescence. We used sadness dysregulation to index ER given findings that depressed individuals show attention biases to, and abnormal processing of, sad stimuli (Garber, Braafladt, & Zeman, n.d.; Gotlib, Krasnoperova, Yue, & Joormann, 2004; Hankin, Gibb, Abela, & Flory, 2010), impacting the regulation of emotions (i.e., sadness). Our second objective was to examine whether corticolimbic rs-FC in preadolescence mediated the hypothesized relationship between ER in school age and depressive symptoms in adolescence. This was assessed by testing three additional research questions: (2) Does ER in school-aged children predict variations in rs-FC of the corticolimbic circuit in preadolescence; (3) do these rs-FC profiles, in turn, predict depressive symptoms in adolescence; and (4) do rs-FC profiles mediate the relationship between ER in school age and depression in adolescence? Based on the literature, we hypothesized that lower ER in school age would be associated with abnormalities in PFC-amygdala rs-FC in preadolescence, which we anticipated would, in turn, predict greater depressive symptoms in adolescence. Finally, we expected PFC-amygdala rs-FC to mediate the relationship between ER in school age and depressive symptoms in adolescence (see Fig. 1).
Schematic of study design. Schematic depicts interrelationships between variable across three developmental time points: school age (S1), preadolescence (S2), and adolescence (S3). Primary measures obtained at each time point are represented by the blue boxes. Secondary measures obtained are represented by the purple boxes. Solid arrows represent direct relationships examined between variables, while the dashed arrow represents the mediated relationship examined between variables. Upper gray arrow represents movement throughout time (and not causal inference). (Color figure online)
Method
Participants
Participants for this study were from the Preschool Depression Study (PDS), a prospective 12-year longitudinal study examining the developmental trajectories of preschool onset depression. Of note, the PDS oversampled for children with or at risk for depression based on current symptoms and history of family and maternal affective disorders (for more details, see Luby, Si, Belden, Tandon, & Spitznagel, 2009). Data acquisition, including neuroimaging and assessment data, is ongoing at the Early Emotional Developmental Program at Washington University School of Medicine in St. Louis, MO. Information regarding recruitment, study parameters (e.g., inclusion and exclusion criteria), and assessment measures have been previously described in Luby et al. (2009). Of relevance, all subjects participating in the neuroimaging arm of the PDS underwent MRI scanning and completed a battery of behavioral assessments. Participants were evaluated in roughly 18-month intervals for a total of three waves. In Scan Waves 1 (S1), 2 (S2), and 3 (S3), participants ranged from 7 to 12, 9 to 14, and 10 to 16 years of age, respectively. Despite some age variation and overlap across scans, we treated each scan wave as a rough approximation of three developmental periods; school age (S1), preadolescence (S2), and adolescence (S3). All study procedures were reviewed and approved by the Institutional Review Board at Washington University School of Medicine. Parents provided written informed consent, while children gave either oral or written assent or consent (depending upon age) following study description.
To examine whether early emotion regulation predicts subsequent connectivity, we focused on emotion regulation assessed at S1, rs-FC connectivity at S2, and depressive symptoms measured at S3 to allow a temporal dissociation in order to test our mediation hypotheses. Thus, the present study included all children that had (1) CSMS data at S1, and (2) usable resting-state scans at S2 (see Fig. 1 for an overview of the study design). A total of 143 participants met inclusion criteria for the current analysis. Participants were divided into groups based on their diagnostic status (for more information, see Diagnostic Measures section): history of MDD (N = 58) and no history of MDD (N = 85). Table 1 provides a summary of relevant demographic and clinical characteristics of this sample.
Diagnostic measures
All participants underwent a diagnostic assessment using the Preschool Age Psychiatric Assessment for children 3–8 years of age (Egger et al., 2006) or the Child and Adolescent Psychiatric Assessment >age 8 (Angold et al., 2009) administered by trained research assistants to assess for psychopathology. Both the PAPA and CAPA are semistructured interviews designed to assess DSM-IV Axis I mental disorders. Both instruments have established reliability and validity (Angold et al., 2009; Egger et al., 2006). Children who met developmentally appropriate diagnostic criteria for major depressive disorder at any time prior to or including S1 were categorized into the MDD group (Luby et al., 2003). Participants were clustered into the No-MDD group if they (1) met diagnostic criteria for clinical disorders other than MDD (including anxiety disorders, ADHD, and conduct disorders) at any point prior to or including S1 or (2) did not meet diagnostic criteria for any clinical disorder (e.g., healthy controls).
Self-report measures
All participants and their parent/legal guardian completed a battery of questionnaires at each scan wave. Two measures of interest were examined for the present study: the Children Sadness Management Scale (CSMS; Zeman, Shipman, & Penza-clyve, 2001) and the Child Depression Inventory–Child Report (CDI-C; Helsel & Matson, 1984). The CSMS assesses children’s ability to manage or regulate their experience with sadness via three dimension scores: Inhibition (overcontrol of sadness), Dysregulated Expression (undercontrol of sadness), and Coping (ability to regulate the intensity and duration of sadness). To evaluate sadness dysregulation, the present study focused on the Dysregulated Expression scale. Greater scores on the Dysregulation Expression scale indicated poorer abilities to modulate sadness. The CDI-C was used to evaluate the severity of depressive symptoms. Psychometric properties for both instruments have been previously established (Knight, Hensley, & Waters, 1988; Smucker, Craighead, Craighead, & Green, 1986; Zeman et al., 2001)
Neuroimaging
All participants completed a battery of neuroimaging scans on a 3-T TIM TRIO scanner at Washington University. This battery included high-resolution structural scans, diffusion-weighted images, and task-based and resting-state functional scans. The present study examined resting-state scans acquired from this battery. Specifically, two resting-state scans; each including 164 frames (~6.8 minutes) were acquired. Participants were instructed to remain awake during scanning, with their eyes closed. Images were acquired using a spin-echo, echo-planar sequence sensitive to BOLD contrast (T2*) (TR = 2500 ms, TE = 27 ms, field of view = 256 mm, flip = 90°, voxel size = 4 × 4 × 4 mm, slices = 36). Additionally, T1-weighted structural images were acquired in the sagittal plane using a magnetization-prepared rapid gradient-echo (MP-RAGE) three-dimensional sequence (TR = 2400 ms, TE = 3.16 ms, flip angle = 8°, 176 slices, field of view = 256 mm, voxel size=1 × 1 × 1 mm). For registration purposes, T2-weighted images were acquired using a 3D-SPACE acquisition (TR = 3200 ms, TE = 497 ms, 160 slices, field of view = 256 mm, voxel size = 1 × 1 × 1 mm).
Preprocessing
All resting-state scans for each participant underwent eight preprocessing steps using an in-house MATLAB script outlined in Power et al. (2014). These steps include (1) image correction for slice-dependent time shifts; (2) removal of the first four images of each resting-state scan to allow BOLD signal to reach steady state; (3) removal of odd/even slice intensity differences due to interpolated acquisition; (4) image realignment within and across scans to reduce rigid body motion; (5) scan intensity normalization to a whole-brain mode value of 1,000; (6) registration of the T1 scan to an atlas template (WU 711-2B) in the Talairach coordinate system using a 12-parameter affine transform and resampled to 1-mm cubic representation; (7) coregistration of the three-dimensional fMRI volume to the T2 and the T2 to the participant’s T1 structural image; and (8) transformation of the fMRI data to 3 × 3 × 3-mm voxel atlas space using a single affine 12-parameter transform.
Functional connectivity processing
The following additional four processing steps were conducted on all rs-FC scans using in-house software (Luking et al., 2011; Sylvester et al., 2013). First, the following nuisance variables were regressed from the BOLD data: average signal from ventricles, white matter, and whole-brain gray matter signal derived from individualized Freesurfer parcellations, as well as six head realignment parameters and their derivatives (24 parameters from Volterra series expansion). Additionally, a temporal band-pass filter (0.009 Hz < f < 0.08 Hz) and spatial smoothing (6 mm full width at half maximum) were applied. Finally, to reduced motion and signal artifact, average global signal and its derivate were regressed out. Scans with excess head motion artifact were censored based on frame-wise displacement values greater than 0.2, as previously described by (Power et al., 2014). Additionally, scan runs with less than 40 frames remaining after censoring and participants with less than 110 total frames remaining across all available runs were excluded from further analyses (N = 59 at S1 and N = 35 at S2). After excess motion scans were identified and censored, all of the above steps were repeated with the raw data (output of the initial preprocessing) interpolating over the censored frames.
Resting-state functional connectivity analyses
Based on the neuroimaging literature on emotion regulation and depression, the present study selected four primary seed ROIs (bilateral dlPFC and bilateral amygdala). These regions have been most consistently associated with ER (Buhle et al., 2014; Uchida et al., 2014) and enable the measurement of cortical–cortical and cortical–subcortical connectivity important to top-down and bottom-up processes involved in ER. Six additional target seeds (bilateral vlPFC, bilateral insula, vmPFC, and dACC) implicated in emotion dysregulation and MDD were selected. Bilateral amygdalae were anatomically defined using Freesurfer’s subcortical parcellations (Pagliaccio et al., 2014). The vmPFC region was created using a spherical ROI 12 mm in diameter and based on Gee et al.’s (2013) coordinates (−3, 35, 1). The vmPFC was selected as a target region given its role in the default mode network (DMN), which is shown to be commonly altered in depression (Chai et al., 2016; Gaffrey et al., 2012; Hamilton, Chen, & Gotlib, 2013; Sheline et al., 2009) The remaining ROIs were also created using spherical ROIs 12 mm in diameter using the FIDL analysis package (http://www.nil.wustl.edu/labs/fidl/index.html) and were based on coordinates from the Buhle et al. (2014) emotion-regulation meta-analysis. The MNI coordinate reported in Buhle et al. (2014) were converted to Tailarach space using an in-house tool based on an affine transformation as reported in Hacker et al. (2013), and Smyser et al. (2016). The seed coordinates were as follows: bilateral dlPFC (±32, 31, 30), dACC (−8, 22, 30), right insula (43, 9, 4), left insula (−36, 16, -1), right vlPFC (47, 24, −4), and left vlPFC (−47, 25, −6). For each participant, we computed the correlation of BOLD time series between each of the four primary seed ROIs (averaging across voxels within the ROIs) to each of the other primary ROIs, as well as the six additional target ROIs. We converted these correlations into Fisher’s r to Z transforms, which were the dependent variables in all subsequent analyses.
Statistical analyses
We conducted a series of linear regressions using R software package. These regressions included age at S1 and gender as covariates, an MDD history (MDD-hx) dummy variable coding for the presence of either a history of MDD or no lifetime history of depression (other diagnosis or healthy), and an interaction term between MDD-Hx and the other predictors of interest to determine if relationships to our independent variables differed as a function of diagnostic status. First, a linear regression was conducted to address whether CSMS scores at S1 predicted CDI scores at S3, using CDI-C scores at S1 and S2 as covariates. Next, to address whether emotion dysregulation in school age predicted rs-FC in preadolescence, we conducted linear regressions on each pairwise correlation at S2—the four seed ROIs to each other and the six target ROIs—using CSMS scores at S1 to predict rs-FC at S2.Footnote 1 To protect against false positives, we applied false discovery rate (FDR) to correct for the number of analyses conducted for each seed region, as shown in Table 2. Of note, FDR was applied to a given seed, rather than across all seeds to obtain a balance between false positives and false negatives. To verify that these CSMS–rs-FC relationships were not a function of concurrent ER, we took all significant regressions in this first step and conducted a follow-up regression to determine whether CSMS scores at S1 predicted rs-FC at S2 above and beyond (when controlling for) concurrent measures of CSMS at S2. Then, to assess connectivity change from S1 to S2, all significant regressions in Step 1 were used to examine whether CSMS at S1 continued to predict rs-FC at S2, controlling for rs-FC at S1 and CSMS at S2 (i.e., residualized change). Next, we examined whether rs-FC at S2 predicted CDI-C scores at S3. Finally, we used PROCESS (Model 4; bootstrap confidence interval) to examine whether significant rs-FC profiles at S2 mediated the relationship between CSMS at S1 and CDI-C scores at S3. These mediation models included additional covariates: CDI-C at S1, CDI-C at S2, and rs-FC at S1. To confirm that psychopathology in the no-MDD group was not attenuating group differences between the MDD and no-MDD groups, post hoc regressions were conducted between children with MDD, other diagnoses (Other-dx; i.e., children with other clinical disorders but not MDD) and healthy children in predicting rs-FC. These post hoc analyses included a, “Other-dx” dummy variable coding for the presence of other clinical disorders, but not MDD (e.g., anxiety and ADHD) and a “CSMS and Other-dx” interaction term. Post hoc regressions were conducted for all significant regressions that survived FDR correction in Step 1 (see Supplemental Materials Table S1).Footnote 2
Results
Clinical characteristics
Table 1 provides a summary of clinical and demographic characteristics. Children with a history of MDD did not differ from children without a history of depression in sex, age, and ethnicity. CSMS at S1 and S3 differed between groups, with children positive for a history of MDD showing higher CSMS scores than children without a history of MDD. A similar trend was found for CSMS at S2. Finally, children with a history of MDD showed significantly higher depressive symptoms at S3 than children without a history of depression.
Does emotion dysregulation in school age predict depressive symptoms in adolescence?
CSMS scores at S1 did not significantly predict CDI-C scores at S3 (p = .596, t = 0.532, B = 0.040) in all children when covarying for the effects of age, sex, and CDI-C at S1 and S2. There were no significant interactions between CSMS at S1 and MDD history in predicting CDI-C at S3, when covarying for the effects of age, sex, and CDI-C scores at S1 and S2. Although a direct relationship between CSMS at S1 and CDI-C at S3 was not observed, subsequent research questions were still examined given evidence suggesting that significant mediators may represent an underpowered direct relationship between two variables (Rucker, Preacher, Tormala, & Petty, 2011; Zhao, Lynch, & Chen, 2010). Thus, in the absence of a direct relationship, it is possible that rs-FC at S2 may mediate a weak association between CSMS at S1 and CDI at S3.
Does emotion regulation in school age predict functional connectivity in preadolescence in children with or without a history of MDD?
Main effect findings
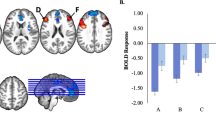
Table 2 provides the average rs-FC values for each pairwise connection as well as the results of the regressions. For clarity purposes, we refer to increased rs-FC as reflecting either stronger positive or weaker negative connectivity and decreased rs-FC as reflecting either stronger negative or weaker positive connectivity. Linear regressions examining whether CSMS at S1 predicted rs-FC at S2 indicated that higher CSMS at S1 predicted increased (e.g., weaker negative) rs-FC between bilateral dlPFC and vmPFC and increased (i.e., stronger positive) rs-FC between bilateral dlPFC and bilateral insula (see Table 2 and Fig. 3). Additionally, higher CSMS scores at S1 significantly predicted decreased (i.e., stronger negative) rs-FC at S2 between the right amygdala with the dACC (see Table 2 and Fig. 2b). These CSMS to rs-FC relationships, with the exception of right amygdala to dACC, survived multiple comparison correction and controlled for the effects of age and sex. Of note, there were no main effect findings for MDD history (see Supplemental Materials Fig. S2 for a graphical illustration of all main effect relationships that survived FDR correction).
Interaction effect findings
There were several significant interactions between CSMS scores and MDD-hx in predicting rs-FC (see Table 2). Relationships between CSMS at S1 and rs-FC at S2 between right DLPFC to dACC, right to left DLPFC, right amygdala to left insula, and left amygdala to right vlPFC significantly differed by MDD history when covarying for age and sex (see Fig. 2b). Of these interactions, the right DLFPC to dACC (illustrated in Fig. 3) survived multiple comparison correction. To further explore the source of this interaction, additional regressions were conducted separately in children with and without a history of MDD. These regressions demonstrated that CSMS at S1 in children with a history of MDD (p = .002, t = 3.11, B = .369), but not in children without depression (p = .648, t = 1.409, B = −.053), predicted increased (i.e., stronger positive) rs-FC between the right dlPFC and dACC at S2 (see Fig. 3).
Childhood emotion regulation predicts functional connectivity in pre-adolescence. Interaction effect of CSMS and right dlPFC to dACC connectivity in children with positive MDD history relative to those without depression. Graph illustrates significant, FDR-corrected relationships. (Color figure online)
Additional covariates
To determine whether the relationships between CSMS at S1 and rs-FC at S2 held above and beyond concurrent emotion dysregulation, CSMS at S2 was added as a covariate (in addition to age and sex) for all regressions that survived multiple comparison. As shown in Table 3, CSMS at S1 continued to predict rs-FC at S2 between bilateral dlPFC-insula and bilateral dlPFC-vmPFC. Additionally, the CSMS × MDD-hx interaction effect remained significant. Further, to determine whether CSMS at S1 predicted rs-FC at S2 even when controlling for rs-FC at S1 (i.e., residualized change from S1 to S2), rs-FC at S1 was used as a covariate (in addition to age and sex) for regressions that survived FDR correction. As shown in Table 4, CSMS at S1 continued to predict increased (i.e., stronger positive) rs-FC between bilateral dlPFC-right insula across diagnostic status. The CSMS × MDD interaction for right dlPFC-dACC at S2 is no longer significant when controlling for right dlPFC-dACC rs-FC at S1.
Does functional connectivity in pre-adolescence predict depressive symptoms in adolescence?
A significant main effect for a relationship between right dlPFC to dACC connectivity at S2 and CDI-C at S3 (p = .0452, t = 2.025, B = .185) was found (covarying for age and sex). Increased right dlPFC-dACC rs-FC (i.e., stronger positive) predicted higher CDI-C scores at S3. However, when CDI-C scores at S2 were added into the regression, dlPFC-dACC connectivity no longer significantly predicted CDI-C scores at S3 (p = 1.904, t = 0.057, B =.145). There were no significant interactions between rs-FC at S2 (identified in Research Question 1) and MDD history in predicting CDI-C scores at S3.
Does rs-FC profiles in preadolescence mediate the relationship between emotion regulation in school age and depressive symptoms in adolescence?
The rs-FC measures at S2 in Table 1 that survived FDR did not mediate the relationship between CSMS at S1 and CDI-C at S3 when controlling for age, sex, CDI-C at S1 and S2, and rs-FC at S1 (see Supplemental Materials Table S3 for mediation results of all rs-FC regions). For a graphical overview of findings for all research questions, see Fig. 4.
Overview of findings for all research questions. CSMS at S1 does not predict CDI at S3 (Research Question 1) but does predict rs-FC profiles at S2 for both main effects and interaction effects (Research Question 2). Only main effects of the right DLPFC-dACC predict CDI at S3 (Research Question 3). Rs-FC profiles at S2 does not mediate the relationship between CSMS at S1 and CDI at S3 (Research Question 4). Arrows represent prediction across time. CSMS = Children Sadness Management Scale; rs-FC = resting-state functional connectivity; CDI = Children Depression Inventory
Specificity analyses
As shown in Supplemental Materials Table S1, there were no significant effects in the Other-dx group (e.g., children with other clinical disorders but not MDD; main effect) and CSMS × Other-dx (an interaction term between CSMS and Other-dx; interaction effect) in predicting rs-FC when controlling for age and sex. To confirm that symptoms of anxiety or disruptive disorders did not better explain variance in predicting rs-FC at S2, separate regressions coding for the presence of anxiety (CSMS × Anxiety) and disruptive disorders (CSMS × Disruptive) were conducted. Results for both sets of regressions showed lower significance levels in predicting right dlPFC-dACC (p = .030 for anxiety and p = .034 for disruptive disorders) when covarying for the effects age and sex. To determine the relative contribution of MDD, anxiety, and disruptive disorders in predicting, CSMS × MDD was subsequently included in a regression model coding for anxiety and disruptive disorders. Findings showed that CSMS × MDD, CSMS × Anxiety, and CSMS × Disruptive were not significant, likely due to collinearity, although effect sizes were higher for CSMS × MDD (see Supplemental Materials Table S4). Taken together, these findings provide evidence consistent with the hypothesis that MDD, over anxiety and disruptive disorders, may be related to variation in dlPFC-dACC connectivity.
Discussion
The present study examined the longitudinal relationships between ER in school age, corticolimbic rs-FC in preadolescence, and depressive symptoms in adolescence in children with and without a history of MDD. Our objectives were to determine whether ER in school age predicted depressive symptoms in adolescence and whether corticolimbic rs-FC in preadolescence mediated this relationship. Specifically, we examined whether (1) ER in school age predicted depressive symptoms in adolescence; (2) ER in school age predicted variations in corticolimbic rs-FC in preadolescence; (3) rs-FC in preadolescence, in turn, predicted depressive symptoms in adolescence; and (4) rs-FC profiles in preadolescence mediated the relationship between ER in school age and depressive symptoms in adolescence (see Fig. 4).
While we hypothesized that lower ER in school age would predict higher depressive symptoms in adolescence, we did not find such a relationship. This finding was surprising given that lower ER have been previously linked to depressive symptoms (Berking et al., 2014; Feng et al., 2009; Silk et al., 2003). Our findings stand in contrast with those of Feng et al. (2009), which showed that lower ER in school age girls (~9 years old) predicted depressive symptoms in preadolescence (~10 years old; Feng et al., 2009). Some important differences may have influenced our ability to replicate Feng et al.’s findings. First, the developmental periods under investigation were different, with Feng et al. measuring depressive symptoms in preadolescence (~age 10 years) and the present study examining depressive symptoms in adolescence (~12–13 years old). This raises the possibility that emotion dysregulation in school age may predict more immediate depressive symptoms, but may be limited in its ability to predict longer-term depressive symptoms. This would explain why ER in preadolescence, but not ER in school age, predicted depressive symptom in adolescence (see Supplemental Materials, Results). Another important difference were baseline CSMS scores; impaired ER during school age in our sample was, on average, higher (~5, higher dysregulation) than in Feng’s sample (~2, lower dysregulation), even though both studies oversampled for depression. The present study also examined both boys and girls, whereas Feng et al. studied only girls. Of note, we did not find interactions with sex predicting depressive symptoms in adolescence (see Supplemental Materials, Results). Overall, our findings did not confirm that emotion dysregulation in school age predicted depressive symptoms in adolescence, suggesting that this risk trajectory of MDD may be more complicated in adolescence.
Although emotion dysregulation in school age did not predict depressive symptoms in adolescence, emotion dysregulation did predict variations in corticolimbic rs-FC in preadolescence. Our findings showed that higher emotion dysregulation in school age predicted weaker negative rs-FC between dlPFC-vmPFC and stronger positive rs-FC between dlPFC-insula in children across diagnostic status. These profiles are consistent with findings reported in ER/depression studies, though we did not find PFC-amygdala rs-FC profile that we originally anticipated. Altered dlPFC–vmPFC connectivity is one of the patterns identified in the Kaiser et al. (2015) meta-analysis and is thought to reflect abnormal communication between executive and default mode networks in depressed adults (Kaiser et al., 2015). Our findings suggest that weaker negative dlPFC-vmPFC rs-FC is also present during preadolescence and that these rs-FC variations may be associated with broader impairments in ER during school age, rather than exclusively in depression. The dlPFC—a region implicated in top-down control functions (Okon-Singer, Hendler, Pessoa, & Shackman, 2015) and cognitive reappraisal (Buhle et al., 2014)—is a key anchor of executive networks (Power et al., 2011) and typically exhibits negative rs-FC with the vmPFC (i.e., as dlPFC activity increases, vmPFC activity decreases; Greicius, Krasnow, Reiss, & Menon, 2003). A weakening in negative dlPFC-vmPFC rs-FC in preadolescence might represent disruptions in executive networks’ ability to execute control over the vmPFC, an area associated with shifting focus from oneself to external environment (Morawetz et al., 2016). It is possible that decoupling in dlPFC-vmPFC rs-FC may be one mechanism by which children with emotion dysregulation allocate disproportionate attentional resources to self-processing. This speculation would be consistent with reports indicating that children and adults with various clinical disorders (Gaffrey et al., 2012; Ho et al., 2015; Sheline et al., 2009) engage more frequently in maladaptive self-focused ER strategies (e.g., rumination; Aldao, Nolen-Hoeksema, & Schweizer, 2010).
Alterations in dlPFC-insula rs-FC are less frequently reported in ER and MDD. Importantly, disruptions in dlPFC-insula rs-FC has been noted in treatment-resistant depressed adults and is thought to contribute to emotion dysregulation (Lui et al., 2011). The insula, which typically exhibits modest positive rs-FC with the dlPFC in adults (Seeley et al., 2007), has been implicated in salience detection (Menon & Uddin, 2010) and recognition of emotion during emotion regulation (Tozzi et al., 2017). Connectivity between frontal cortices and anterior insula is thought to play a role in activating executive network structures (i.e., dlPFC) and deactivating default mode structures (i.e., vmPFC; Sridharan, Levitin, & Menon, 2008), though perhaps more strongly in adults than in children (Uddin, Supekar, Ryali, & Menon, 2011). One speculation for stronger positive dlPFC-insula rs-FC in preadolescence is that children who experience greater emotion dysregulation might activate the insula more frequently, as is seen in emotionally dysregulated adults (Picó-Pérez, Radua, Steward, Menchón, & Soriano-Mas, 2017), potentially intensifying coupling to the dlPFC and disrupting the balance between salience detection (insula), self-referential processing (vmPFC), and emotion regulation (dlPFC).
Of note, these rs-FC profiles (i.e., dlPFC-vmPFC and dlPFC-insula) did not interact with history of depression, suggesting that variations in these profiles are characteristic of emotion dysregulation more broadly. This is not surprising, given that emotion dysregulation is transdiagnotstic(Hofmann, Sawyer, Fang, & Asnaani, 2012), with disorders such as anxiety disorders also reporting rs-FC alterations in dlPFC, vmPFC, and insula (Miller et al., 2017; Taylor & Liberzon, 2007; Young et al., 2017).
Lastly, ER in school age predicted stronger positive dlPFC-dACC rs-FC in preadolescence, but only in children with a history of depression. This finding is consistent with disruptions in dlPFC-dACC reported in children at risk for depression (Chai et al., 2016) and depressed adults (Aizenstein et al., 2010; Ye et al., 2012). The dACC typically shows weak positive connectivity with the dlPFC (Koski & Paus, 2000; Margulies et al., 2007) and is hypothesized to be involved in the maintenance of control signals (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008). The dACC and dlPFC are each part of broader executive system important for top-down control processes (Dosenbach et al., 2007), including emotion regulation in children (Joormann, Cooney, Henry, & Gotlib, 2012) and adults (Ochsner et al., 2012). It is possible that increased coupling between dlPFC and dACC might reflect disruptions in the signaling, maintenance, or execution of top-down processes, which appear to be more prominent in children with a history of depression.
Of note, the relationships between school-age ER and all rs-FC profiles (i.e., dlPFC-vmPFC, dlPFC-insula, and dlPFC-dACC) remained significant after controlling for concurrent emotion dysregulation, suggesting that variations in rs-FC did not merely reflect emotion dysregulation in preadolescence. It is also worth noting that dlPFC-vmPFC rs-FC in children across diagnosis and dlPFC-dACC rs-FC in children with a history of depression did not survive significance when controlling for rs-FC in school age. This suggests that emotion dysregulation predicted rs-FC in preadolescence but not connectivity change from school age to preadolescence. Further, while not a goal of the study, one interesting observation was that MDD history did not directly predict these rs-FC profiles, with the exception of right dlPFC-dACC. However, given that depressed children also tend to show impaired ER (Garber & Dodge, 1991; McLaughlin, Hatzenbuehler, Mennin, & Nolen-Hoeksema, 2011), it is possible that the relationship between emotion dysregulation and rs-FC is capturing variance that would otherwise be attributed to MDD history. Teasing apart the variances accounted for by emotion dysregulation and MDD in even larger longitudinal sample is an area open for future research.
Importantly, contrary to our hypothesis, we did not find that emotion dysregulation in school age predicted PFC-amygdala rs-FC in preadolescence. This was surprising given that altered amygdala rs-FC has been frequently linked to MDD (Anand et al., 2005; Luking et al., 2011) and emotion dysregulation across development (Bebko et al., 2015; Morawetz et al., 2016; Veer et al., 2010). A few modest relationships with the amygdala were identified, but they did not survive FDR correction (see Table 2). One explanation is that altered PFC-amygdala rs-FC might be more strongly apparent during explicit demands of ER such as reappraisal (Murphy et al., 2016). Another possibility is that amygdala rs-FC might vary more strongly with brain regions other than those examined in this investigation (e.g., hippocampus; Cullen et al., 2014).
We hypothesized that rs-FC identified in preadolescence (predicted by ER in school age) would, in turn, predict depressive symptoms in adolescence. Our findings showed that stronger positive right dlPFC-dACC rs-FC in preadolescence predicted higher depressive symptoms in adolescence in children across diagnostic history groups (see Fig. 4). This finding is interesting given that the relationship between ER in school age and dlPFC-dACC rs-FC in preadolescence was stronger children with a history of MDD. However, dlPFC-dACC rs-FC at preadolescence no longer predicted depression in adolescence when controlling for concurrent preadolescent depression. This suggests that dlPFC-dACC rs-FC does not predict change in depression from preadolescence to adolescence. Finally, increased dlPFC-dACC rs-FC in preadolescence did not mediate the relationship between emotion dysregulation in school age and depressive symptoms in adolescence. The lack of mediation is consistent with our finding that ER in school age did not predict depressive symptoms in adolescence, although this later finding is at odds with evidence indicating a relationship between ER deficits and MDD (Berking et al., 2014; Feng et al., 2009; Joormann & Gotlib, 2010; Joormann & Stanton, 2016; McLaughlin et al., 2011; Wang et al., 2008). It is possible that other rs-FC profiles might be more significant mediators, such as ones not examined in the current study (e.g., insula-amygdala rs-FC; Veer et al., 2010). Overall, our findings highlight the need to investigate additional mediating and moderating variables in order to better understand the neural mechanisms of the relationship between emotion dysregulation and depression across development.
These findings should be considered in light of several limitations. First, the absence of cognitive control measures precludes us from determining whether these rs-FC profiles are directly related to impairments in executive functions important to ER. Further, the present study used a self-report measure of ER, raising a potential constraint in our ability to objectively capture ER deficits. Another limitation is the overlap in age across school age, preadolescence, and adolescence, which may have limited our ability to identify clearly defined relationships from one developmental period to another. Further, the onset of puberty, occurring roughly during preadolescence, is known to affect the trajectories of rs-FC (e.g., vmPFC-amygdala; Gee et al., 2013), particularly in cognitive control regions important for ER (McRae et al., 2012). Thus, changes during preadolescence may also obscure the detection of well-defined relationships across development. It is important to note, however, that age was used as a covariate for all regressions, mediation, and post hoc analyses. It is also important to consider that FDR correction was conducted within-ROI rather than across ROIs—a method that may have yielded findings that might not have survived across-ROI correction. A final limitation is that the present study oversampled for early onset depression, possibly limiting the generalizability of our findings.
Conclusions
In summary, we did not find a relationship between emotion dysregulation in school age and depressive symptoms in adolescence. Furthermore, rs-FC did not mediate this hypothesized relationship. However, our findings demonstrate that greater emotion dysregulation in school age predicted alterations in dlPFC connectivity with the dACC and insula in children with and without a history of MDD during preadolescence, as well as between dlPFC-dACC in children with a history of depression. These profiles are consistent with the hypothesis that emotion dysregulation is associated with abnormalities in top-down control functions. The extent to which these relationships might confer greater risk for depression in adolescence remains unclear. Future work examining the role of control networks in emotion regulation throughout development will be critical to understanding the neural mechanisms by which emotion dysregulation might contribute to depression.
Notes
We also examined whether rs-FC at S1 predicted variation in ER at S2. Upon applying FDR correction for a given seed, we found that only left dlPFC-right amygdala rs-FC predicted ER at S2 (p = .044). Specifically, increase connectivity (e.g., weaker negative connectivity) predicted higher CSMS scores.
One outlier in CDI-C scores was identified. Thus, all regressions, mediation, and post hoc analyses that included CDI-C scores were reanalyzed. All findings held. Therefore, the outlier was retained in all reported findings.
References
Aizenstein, H., Butters, M., Wu, M., Mazurkewicz, L., Stenger, A., Gianaros, P.,... Carter, C. S. (2010). Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. American Journal of Geriatric Psychiatry, 17(1), 30–42. doi:https://doi.org/10.1097/JGP.0b013e31817b60af.Altered
Aldao, A., Nolen-Hoeksema, S., & Schweizer, S. (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. doi:https://doi.org/10.1016/j.cpr.2009.11.004
Anand, A., Li, Y., Wang, Y., Wu, J., Gao, S., Bukhari, L.,. ... Lowe, M. J. (2005). Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biological Psychiatry, 57(10), 1079–1088. doi:https://doi.org/10.1016/j.biopsych.2005.02.021
Angold, A., Prendergast, M., Cox, A., Harrington, R., Simonoff, E., & Rutter, M. (2009). The Child and Adolescent Psychiatric Assessment (CAPA). Psychological Medicine, 25(4), 739. doi:https://doi.org/10.1017/S003329170003498X
Avenevoli, S., Swendsen, J., He, J. P., Burstein, M., & Merikangas, K. R. (2015). Major depression in the national comorbidity survey- adolescent supplement: Prevalence, correlates, and treatment. Journal of the American Academy of Child and Adolescent Psychiatry, 54(1), 37–44. doi:https://doi.org/10.1016/j.jaac.2014.10.010
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. doi:https://doi.org/10.1093/scan/nsm029
Bebko, G., Bertocci, M., Chase, H., Dwojak, A., Bonar, L., Almeida, J.,... Phillips, M. L. (2015). Decreased amygdala–insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Research: Neuroimaging, 231(1), 77–86. doi:https://doi.org/10.1016/j.pscychresns.2014.10.015
Belden, A. C., Pagliaccio, D., Murphy, E. R., Luby, J. L., & Barch, D. M. (2015). Neural activation during cognitive emotion regulation in previously depressed compared to healthy children: Evidence of specific alterations. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 771–781. doi:https://doi.org/10.1016/j.jaac.2015.06.014
Berking, M., Wirtz, C. M., Svaldi, J., & Hofmann, S. G. (2014). Emotion regulation predicts symptoms of depression over five years. Behaviour Research and Therapy, 57, 13–20. doi:https://doi.org/10.1016/j.brat.2014.03.003
Buhle, J. T., Silvers, J. A., Wage, T. D., Lopez, R., Onyemekwu, C., Kober, H.,. .. Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. doi:https://doi.org/10.1093/cercor/bht154
Burghy, C. A., Stodola, D. E., Ruttle, P. L., Molloy, E. K., Jeffrey, M., Oler, J. A., ... Birn, R. M. (2013). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. doi:https://doi.org/10.1038/nn.3257
Chai, X. J., Hirshfeld-Becker, D., Biederman, J., Uchida, M., Doehrmann, O., Leonard, J. A.,. ... Whitfield-Gabrieli, S. (2016). Altered intrinsic functional brain architecture in children at familial risk of major depression. Biological Psychiatry, 80(11), 849–858. doi:https://doi.org/10.1016/j.biopsych.2015.12.003
Chattopadhyay, S., Tait, R., Simas, T., van Nieuwenhuizen, A., Hagan, C. C., Holt, R. J., ... Suckling, J. (2017). Cognitive behavioral therapy lowers elevated functional connectivity in depressed adolescents. EBioMedicine, 17, 216–222. doi:https://doi.org/10.1016/j.ebiom.2017.02.010
Connolly, C. G., Ho, T. C., Blom, E. H., LeWinn, K. Z., Sacchet, M. D., Tymofiyeva, O.,... Yang, T. T. (2017). Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of Affective Disorders, 207, 86–94. doi:https://doi.org/10.1016/j.jad.2016.09.026
Cullen, K. R., Westlund, M. K., Klimes-Dougan, B., Mueller, B. A., Houri, A., Eberly, L. E., & Lim, K. O. (2014). Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. doi:https://doi.org/10.1001/jamapsychiatry.2014.1087
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. doi:https://doi.org/10.1016/j.tics.2008.01.001
Dosenbach, N. U. F., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A. T., ... Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. doi:https://doi.org/10.1073/pnas.0704320104
Egger, H. L., Erkanli, A., Keeler, G., Potts, E., Walter, B. K., & Angold, A. (2006). Test–retest reliability of the Preschool Age Psychiatric Assessment (PAPA). Journal of the American Academy of Child and Adolescent Psychiatry, 45(5), 538–549. doi:https://doi.org/10.1016/S0084-3970(08)70314-1
Feng, X., Keenan, K., Hipwell, A. E., Henneberger, A. K., Rischall, M. S., Butch, J., Babinski, D. E. (2009). Longitudinal associations between emotion regulation and depression in preadolescent girls: Moderation by the caregiving environment, 45(3), 798–808. doi:https://doi.org/10.1037/a0014617
Frank, D. W., Dewitt, M., Hudgens-Haney, M., Schaeffer, D. J., Ball, B. H., Schwarz, N. F., ... Sabatinelli, D. (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. doi:https://doi.org/10.1016/j.neubiorev.2014.06.010
Gaffrey, M. S., Luby, J. L., Botteron, K., Repovš, G., & Barch, D. M. (2012). Default mode network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(9), 964–972. doi:https://doi.org/10.1111/j.1469-7610.2012.02552.x
Gaffrey, M. S., Luby, J. L., Repovš, G., Belden, A. C., Botteron, K. N., Luking, K. R., & Barch, D. M. (2010). Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport, 21(18), 1182–1188. doi:https://doi.org/10.1097/WNR.0b013e32834127eb
Garber, J., Braafladt, N., & Zeman, J. (n.d.). The regulation of sad affect: An information-processing perspective. In J. Garber & K. A. Dodge (Eds.), The development of emotion regulation and dysregulation (pp. 208–240). Cambridge, UK: Cambridge University Press. doi:https://doi.org/10.1017/CBO9780511663963.011
Garber, J., & Dodge, K. A. (1991). The development of emotion regulation and dysregulation. Cambridge, UK: Cambridge University Press.
Garnefski, N., Kraaij, V., & van Etten, M. (2005). Specificity of relations between adolescents’ cognitive emotion regulation strategies and Internalizing and externalizing psychopathology. Journal of Adolescence, 28(5), 619–631. doi:https://doi.org/10.1016/j.adolescence.2004.12.009
Garnefski, N., & Kraaij, V. (2006). Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences, 33(8), 659–1669. doi:https://doi.org/10.1016/j.paid.2005.12.009
Gee, D. G., Humphreys, K. L., Flannery, J., Goff, B., Telzer, E. H., Shapiro, M., Tottenham, N. (2013). A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. doi:https://doi.org/10.1523/JNEUROSCI.3446-12.2013
Gotlib, I. H., Krasnoperova, E., Yue, D. N., & Joormann, J. (2004). Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology, 113(1), 127–135. doi:https://doi.org/10.1037/0021-843X.113.1.121
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. doi:https://doi.org/10.1073/pnas.0135058100
Hacker, C. D., Laumann, T. O., Szrama, N. P., Baldassarre, A., Snyder, A. Z., Leuthardt, E. C., & Corbetta, M. (2013). Resting state network estimation in individual subjects. NeuroImage, 82, 616–633. doi:https://doi.org/10.1016/j.neuroimage.2013.05.108
Hamilton, J. P., Chen, M. C., & Gotlib, I. H. (2013). Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiology of Disease, 52, 4–11. doi:https://doi.org/10.1016/j.nbd.2012.01.015
Hankin, B. L., Gibb, B. E., Abela, J. R. Z., & Flory, K. (2010). Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology, 119(3), 491–501. doi:https://doi.org/10.1037/a0019609
Helsel, W. J., & Matson, J. L. (1984). The assessment of depression in children: The internal structure of the Child Depression Inventory (CDI). Behaviour Research and Therapy, 22(3), 289–298. doi:https://doi.org/10.1016/0005-7967(84)90009-3
Ho, T. C., Connolly, C. G., Henje Blom, E., LeWinn, K. Z., Strigo, I. A., Paulus, M. P.,... Yang, T. T. (2015). Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry, 78(9), 635–646. doi:https://doi.org/10.1016/j.biopsych.2014.09.002
Hofmann, S. G., Sawyer, A. T., Fang, A., & Asnaani, A. (2012). Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety, 29(5), 409–416. doi:https://doi.org/10.1002/da.21888
Joormann, J., & Gotlib, I. H. (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition & Emotion, 24(2), 281–298. doi:https://doi.org/10.1080/02699930903407948
Joormann, J., Cooney, R. E., Henry, M. L., & Gotlib, I. H. (2012). Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology, 121(1), 61–72. doi:https://doi.org/10.1037/a0025294
Joormann, J., & Quinn, M. E. (2014). Cognitive processes and emotion regulation in depression. Depression and Anxiety, 31(4), 308–315. doi:https://doi.org/10.1002/da.22264
Joormann, J., & Stanton, C. (2016). Examining emotion regulation in depression: A review and future directions. Behaviour Research and Therapy, 86, 35–49. doi:https://doi.org/10.1016/j.brat.2016.07.007
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., & Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry, 2478(6), 603–611. doi:https://doi.org/10.1001/jamapsychiatry.2015.0071
Knight, D., Hensley, V. R., & Waters, B. (1988). Validation of the Children’s Depression Scale and the Children’s Depression Inventory in a prepubertal sample. Journal of Child Psychology and Psychiatry, 29(6), 853–863. doi:https://doi.org/10.1111/j.1469-7610.1988.tb00758.x
Koski, L., & Paus, T. (2000). Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain-mapping meta-analysis. Experimental Brain Research, 133, 55–65. doi:https://doi.org/10.1007/s002210000400
Koster, E. H. W., De Lissnyder, E., Derakshan, N., & De Raedt, R. (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31(1), 138–145. doi:https://doi.org/10.1016/j.cpr.2010.08.005
Lois, G., & Wessa, M. (2016). Differential association of default mode network connectivity and rumination in healthy individuals and remitted MDD patients. Social Cognitive and Affective Neuroscience, 11(11), 1792–1801. doi:https://doi.org/10.1093/scan/nsw085
Luby, J. L., Heffelfinger, A. K., Mrakotsky, C., Brown, K. M., Hessler, M. J., Wallis, J. M., & Spitznagel, E. L. (2003). The clinical picture of depression in preschool children. Journal of the American Academy of Child and Adolescent Psychiatry, 42(3), 340–348. doi:https://doi.org/10.1097/00004583-200303000-00015
Luby, J. L., Si, X., Belden, A. C., Tandon, M., & Spitznagel, E. (2009). Preschool depression: Homotypic continuity and course over 24 months. Archives of General Psychiatry, 66(8), 897–905. doi:https://doi.org/10.1001/archgenpsychiatry.2009.97
Lui, S., Wu, Q., Qiu, L., Yang, X., Kuang, W., Chan, R. C. K., ... Gong, Q. (2011). Resting-state functional connectivity in treatment-resistant depression. American Journal of Psychiatry, 168(6), 642–648. doi:https://doi.org/10.1176/appi.ajp.2010.10101419
Luking, K. R., Repovs, G., Belden, A. C., Gaffrey, M. S., Botteron, K. N., Luby, J. L., & Barch, D. M. (2011). Functional connectivity of the amygdala in early-childhood-onset depression. Journal of the American Academy of Child and Adolescent Psychiatry, 50(10), 1027–1041. doi:https://doi.org/10.1016/j.jaac.2011.07.019
Margulies, D. S., Kelly, A. M. C., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–588. doi:https://doi.org/10.1016/j.neuroimage.2007.05.019
McLaughlin, K. A., Hatzenbuehler, M. L., Mennin, D. S., & Nolen-Hoeksema, S. (2011). Emotion dysregulation and adolescent psychopathology: A prospective study. Behaviour Research and Therapy, 49(9), 544–554. doi:https://doi.org/10.1016/j.brat.2011.06.003
McRae, K., Gross, J. J., Weber, J., Robertson, E. R., Sokol-Hessner, P., Ray, R. D.,... Ochsner, K. N. (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. doi:https://doi.org/10.1093/scan/nsr093
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5/6), 655–667. doi:https://doi.org/10.1007/s00429-010-0262-0
Merikangas, K. R., He, J. P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., ... Swendsen, J. (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. doi:https://doi.org/10.1016/j.jaac.2010.05.017
Miller, D. R., Hayes, S. M., Hayes, J. P., Spielberg, J. M., Lafleche, G., & Verfaellie, M. (2017). Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(4), 363–371. doi:https://doi.org/10.1016/j.bpsc.2016.12.006
Morawetz, C., Kellermann, T., Kogler, L., Radke, S., Blechert, J., & Derntl, B. (2016). Intrinsic functional connectivity underlying successful emotion regulation of angry faces. Social Cognitive and Affective Neuroscience. doi:https://doi.org/10.1093/scan/nsw107
Murphy, E. R., Barch, D. M., Pagliaccio, D., Luby, J. L., & Belden, A. C. (2016). Functional connectivity of the amygdala and subgenual cingulate during cognitive reappraisal of emotions in children with MDD history is associated with rumination. Developmental Cognitive Neuroscience, 18, 86–100. doi:https://doi.org/10.1016/j.dcn.2015.11.003
Ochsner, K. N., Silvers, J. A., & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. doi:https://doi.org/10.1111/j.1749-6632.2012.06751.x
Okon-Singer, H., Hendler, T., Pessoa, L., & Shackman, A. J. (2015). The neurobiology of emotion cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience, 9, 58. doi:https://doi.org/10.3389/fnhum.2015.00058
Pagliaccio, D., Luby, J. L., Bogdan, R., Agrawal, A., Gaffrey, M. S., Belden, A. C.,... Barch, D. M. (2014). Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology, 39(5), 1245–1253. doi:https://doi.org/10.1038/npp.2013.327
Pannekoek, J. N., van der Werff, S. J. A., Meens, P. H. F., van den Bulk, B. G., Jolles, D. D., Veer, I. M., ... Vermeiren, R. R. J. M. (2014). Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naïve clinically depressed adolescents. Journal of Child Psychology and Psychiatry, 55(12), 1317–1327. doi:https://doi.org/10.1111/jcpp.12266
Peters, A. T., Burkhouse, K., Feldhaus, C. C., Langenecker, S. A., & Jacobs, R. H. (2016). Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. Journal of Affective Disorders, 200, 178–181. doi:https://doi.org/10.1016/j.jad.2016.03.059
Peeters, F., Nicolson, N. A., Berkhof, J., Delespaul, P., & deVries, M. (2003). Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology, 112(2), 203–211. doi:https://doi.org/10.1037/0021-843X.112.2.203
Picó-Pérez, M., Radua, J., Steward, T., Menchón, J. M., & Soriano-Mas, C. (2017). Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79, 96–104. doi:https://doi.org/10.1016/j.pnpbp.2017.06.001
Pitskel, N. B., Bolling, D. Z., Kaiser, M. D., Crowley, M. J., & Pelphrey, K. A. (2011). How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Developmental Cognitive Neuroscience, 1(3), 324–337. doi:https://doi.org/10.1016/j.dcn.2011.03.004
Power, J. D., Cohen, A. L., Nelson, S. S. M., Wig, G. S., Barnes, K. A., Church, J. A., ... Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi:https://doi.org/10.1016/j.neuron.2011.09.006
Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). NeuroImage methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. doi:https://doi.org/10.1016/j.neuroimage.2013.08.048
Rive, M. M., van Rooijen, G., Veltman, D. J., Phillips, M. L., Schene, A. H., & Ruhé, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder: A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2529–2553. doi:https://doi.org/10.1016/j.neubiorev.2013.07.018
Rucker, D. D., Preacher, K. J., Tormala, Z. L., & Petty, R. E. (2011). Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass, 5(6), 359–371. doi:https://doi.org/10.1111/j.1751-9004.2011.00355.x
Schiller, D., & Delgado, M. R. (2010). Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in Cognitive Sciences, 14(6), 268–276. doi:https://doi.org/10.1016/j.tics.2010.04.002
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H.,... Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi:https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seminowicz, D., Mayberg, H., McIntosh, A., Goldapple, K., Kennedy, S., Segal, Z., & Rafi-Tari, S. (2004). Limbic–frontal circuitry in major depression: A path modeling metanalysis. NeuroImage, 22(1), 409–418. doi:https://doi.org/10.1016/j.neuroimage.2004.01.015
Sheline, Y. I., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z.,... Raichle, M. E. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–1947. doi:https://doi.org/10.1073/pnas.0812686106
Siener, S., & Kerns, K. A. (2012). Emotion regulation and depressive symptoms in preadolescence. Child Psychiatry and Human Development, 43(3), 414–430. doi:https://doi.org/10.1007/s10578-011-0274-x
Silk, J. S., Steinberg, L., & Morris, A. S. (2003). Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development, 74(6), 1869–1880. doi:https://doi.org/10.1046/j.1467-8624.2003.00643.x
Smucker, M. R., Craighead, W. E., Craighead, L. W., & Green, B. J. (1986). Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology, 14(1), 25–39. doi:https://doi.org/10.1007/BF00917219
Smyser, C. D., Dosenbach, N. U. F., Smyser, T. A., Snyder, A. Z., Rogers, C. E., Inder, T. E., ... Neil, J. J. (2016). Prediction of brain maturity in infants using machine-learning algorithms. NeuroImage, 136, 1–9. doi:https://doi.org/10.1016/j.neuroimage.2016.05.029
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–12574. doi:https://doi.org/10.1073/pnas.0800005105
Stephanou, K., Davey, C. G., Kerestes, R., Whittle, S., & Harrison, B. J. (2017).Hard to look on the bright side: Neural correlates of impaired emotion regulation in depressed youth. Social Cognitive and Affective Neuroscience, 12(7)1138–1148. doi:https://doi.org/10.1093/scan/nsx039
Sylvester, C. M., Barch, D. M., Corbetta, M., Power, J. D., Schlaggar, B. L., & Luby, J. L. (2013). Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 52(12), 1326–1336. doi:https://doi.org/10.1016/j.jaac.2013.10.001
Taylor, S. F., & Liberzon, I. (2007). Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences, 11(10), 413–418. doihttps://doi.org/10.1016/j.tics.2007.08.006
Tozzi, L., Doolin, K., Farrel, C., Joseph, S., O’Keane, V., & Frodl, T. (2017). Functional magnetic resonance imaging correlates of emotion recognition and voluntary attentional regulation in depression: A generalized psycho-physiological interaction study. Journal of Affective Disorders, 208, 535–544. doi:https://doi.org/10.1016/j.jad.2016.10.029
Uchida, M., Biederman, J., Gabrieli, J. D. E., Micco, J., De Los Angeles, C., Brown, A.,... Whitfield-Gabrieli, S. (2014). Emotion regulation ability varies in relation to intrinsic functional brain architecture. Social Cognitive and Affective Neuroscience, 10(12), 1738–1748. doi:https://doi.org/10.1093/scan/nsv059
Uddin, L., Supekar, K., Ryali, S., & Menon, V. (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of Neuroscience, 31(50), 18578–18589. doi:https://doi.org/10.1523/JNEUROSCI.4465-11.2011
van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. doi:https://doi.org/10.1016/j.euroneuro.2010.03.008
Veer, I. M., Beckmann, C. F., van Tol, M.-J., Ferrarini, L., Milles, J., Veltman, D. J., ... Rombouts, S. A. R. B. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience, 4, 1–10. doi:https://doi.org/10.3389/fnsys.2010.00041
Wang, L., LaBar, K. S., Smoski, M., Rosenthal, M. Z., Dolcos, F., Lynch, T. R.,... McCarthy, G. (2008). Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research–Neuroimaging, 163(2), 143–155. doi:https://doi.org/10.1016/j.pscychresns.2007.10.004
Wiens, K., Williams, J. V. A., Lavorato, D. H., Duffy, A., Pringsheim, T. M., Sajobi, T. T., & Patten, S. B. (2017). Is the prevalence of major depression increasing in the Canadian adolescent population? Assessing trends from 2000 to 2014. Journal of Affective Disorders, 210, 22–26. doi:https://doi.org/10.1016/j.jad.2016.11.018
Winecoff, A., Clithero, J. A., Carter, R. M., Bergman, S. R., Wang, L., & Huettel, S. A. (2013). Ventromedial prefrontal cortex encodes emotional value. Journal of Neuroscience, 33(27), 11032–11039. doi:https://doi.org/10.1523/JNEUROSCI.4317-12.2013
World Health Organization. (2016). Depression. Retrieved from http://www.who.int/mediacentre/factsheets/fs369/en/#.WGvG1JXw-cQ.mendeley
Ye, T., Peng, J., Nie, B., Gao, J., Liu, J., Li, Y.,... Shan, B. (2012). Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. European Journal of Radiology, 81(12), 4035–4040. doi:https://doi.org/10.1016/j.ejrad.2011.04.058
Young, K. S., Burklund, L. J., Torre, J. B., Saxbe, D., Lieberman, M. D., & Craske, M. G. (2017). Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Research: NeuroImaging, 261, 44–51. doi:https://doi.org/10.1016/j.pscychresns.2017.01.005
Zeman, J., Shipman, K., & Penza-clyve, S. (2001). Development and initial validation of the Children’s Sadness Management Scale. Journal of Nonverbal Behavior, 25(3), 187–205. doi:https://doi.org/10.1023/A:1010623226626
Zhao, X., Lynch, J. G., & Chen, Q. (2010). Reconsidering Baron and Kenny: Myths and Truths about Mediation Analysis. Journal of Consumer Research, 3(7), (2), 197–206.. doi:https://doi.org/10.1086/6512577
Zhu, X., Wang, X., Xiao, J., Liao, J., Zhong, M., Wang, W., & Yao, S. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry, 71(7), 611–617. doi:https://doi.org/10.1016/j.biopsych.2011.10.035
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Interest
All authors report no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 986 kb)
Rights and permissions
About this article
Cite this article
Lopez, K.C., Luby, J.L., Belden, A.C. et al. Emotion dysregulation and functional connectivity in children with and without a history of major depressive disorder. Cogn Affect Behav Neurosci 18, 232–248 (2018). https://doi.org/10.3758/s13415-018-0564-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0564-x