Abstract
The influence of top-down attentional control on the selection of salient visual stimuli has been examined extensively. Some accounts suggest all salient stimuli capture attention in a stimulus-driven manner, while others suggest salient stimuli capture attention contingent on top-down relevance. Evidence consistently shows target templates allow only salient stimuli sharing a target’s features to capture attention, while salient stimuli not sharing a target’s features do not. A number of hypotheses (e.g., contingent orienting, disengagement, signal suppression) from both sides of this debate have been proposed; however, most predict similar performance in the visual search and spatial cuing tasks. The present study combined a cuing task, in which subjects identified a target defined by its having a unique feature, with a probe identification task developed by Gaspelin, Leonard, and Luck (Psychological Science, 26, 1740-1750, 2015), in which subjects identified letters appearing in potential target locations just after the appearance of a salient cue that matched or did not match the target-defining feature. The probe task provided a measure of where attention was focused just after the cue’s appearance. In six experiments, we observed top-down modulation of spatial cuing effects in response times and probe identification: Probes in the cued location were identified more often, but more when preceded by a cue that shared the target-defining feature. Though not unequivocal, the results are explained in terms of the on-going debate over whether top-down attentional control can prevent bottom-up capture by salient, task-irrelevant stimuli.
Similar content being viewed by others
Introduction
Over the past 25 years, an abundance of research has examined how top-down control interacts with bottom-up signals from the environment for the allocation of attention to items and locations (for reviews see, Burnham, 2007; Lamy, Leber, & Egeth, 2012; Theeuwes, 2010; Wright & Ward, 2008; Yantis, 2000). Salient objects in the visual field capture attention in a seemingly automatic or stimulus-driven manner (e.g., Belopolsky, Schreij, & Theeuwes, 2010; Theeuwes, 1992, 1994; Theeuwes, Atchley, & Kramer, 2000; Turatto & Galfano, 2001; Turatto, Galfano, Gardini, & Mascetti, 2004; Yantis & Egeth, 1999). However, research shows an observer’s attentional control settings modulate which salient items capture attention (e.g., Anderson & Folk, 2010; Ansorge & Heumann, 2003; Folk & Remington, 1998, 2006; Folk, Remington, & Johnston, 1992; Folk, Remington & Wright, 1994; Gibson & Kelsey, 1998; Lamy & Egeth, 2003; Lamy, Leber, & Egeth, 2004; Leber & Egeth, 2006). Top-down modulation of attention is well established, and different hypotheses have been proposed to account for the finding that task-relevant stimuli capture attention while salient and task-irrelevant stimuli do not, or elicit smaller attentional capture. This study employed a probe-detection approach to determine where visual attention focused following the appearance of salient task-relevant and task-irrelevant stimuli. Across several experiments, this allowed us to examine the influence of top-down settings on the early allocation of attention to salient items in the visual field.
Attentional capture debate
The sudden appearance of an object in the periphery can trigger an exogenous shift of attention, resulting in faster response times (RTs) and greater accuracy to targets in the same spatial location than in a different location (e.g., Franconeri & Simons & Junge, 2004; Jonides, 1981; Posner, 1980; Pratt, Sekuler, & McAuliffe, 2001; Theeuwes, 1994; Wright & Ward, 2008; Yantis & Jonides, 1984). For example, when a peripheral onset appears, responding is facilitated when a target appears in the same cued location (valid cue) than some other uncued location (invalid cue).
Folk et al. (1992) found that salient items capture attention contingent on their feature relevance or similarity to a searched-for target. In their study, a red cue or an onset cue preceded a target defined by its being the onset of a single item, or a red item among white items. Folk et al. (1992) hypothesized that subjects would enter the target-defining feature into an attentional set, which would moderate shifting attention toward the cues. Specifically, cues featurally similar to the target (target-relevant cues) would capture attention, but featurally dissimilar cues (target-irrelevant cues) would not. Consistent with this contingent orienting hypothesis, only the red cue elicited a cuing effect when observers were set to detect red targets, and only the onset cue elicited a cuing effect when observers were set to detect onsets; hence, the bottom-up signals interacted with the observers’ top-down settings.
Studies have consistently observed similar interactions between target features and cue features (e.g., Anderson, 2014; Anderson & Folk, 2010, 2012; Ansorge & Heumann, 2003; Bacon & Egeth, 1994; Barras & Kerzel, 2016; Born, Kerzel, & Pratt, 2015; Chen & Mordkoff, 2007; Folk & Remington, 1998, 2006, 2008, 2010; Folk et al., 1992, 1994; Gibson & Kelsey, 1998; Goller, Ditye, & Ansorge, 2016; Lamy, Leber, & Egeth, 2003; Leber & Egeth, 2006; Lien, Ruthruff, & Cornett, 2010; Lien, Ruthruff, Goodin, & Remington, 2008; Lien, Ruthruff, & Johnston, 2010). For example, Gibson and Kelsey (1998; Johnson, Hutchison, & Neill, 2001) demonstrated how the global features of a target display, not just a target-defining feature, modulated attentional capture. Anderson and Folk (2010) found cuing effects to vary as a function of a cue-target similarity; specifically, the redder (or greener) a cue was to a red (or green) target, the larger the cuing effect. It is also possible for several distinct colors to be maintained in the attentional set, and any target-relevant cue will elicit a cuing effect (Irons, Folk, & Remington, 2012; Pratt & McAuliffe, 2002). Studies have also shown contingent capture of attention in RSVP tasks, reflecting both spatial capture (Folk, Leber, & Egeth, 2002) and non-spatial capture (Folk, Leber, & Egeth, 2006). Additionally, only target-relevant cues elicit an N2pc (e.g., Lien et al., 2010) – a neural marker of attention shifting to a peripheral stimulus. Specifically, the N2pc is an increased negativity contralateral to an attended object that is seen at posterior recording sites, 200–300 ms after an object has been presented (Eimer, 1996; Luck, 2006; Luck & Hillyard, 1994a, 1994b). In short, an abundance of research has shown how top-down settings interact with bottom-up signals and only task-relevant stimuli capture attention.
Nonetheless, this has not been without controversy, and many studies claim to show evidence of stimulus-driven capture by salient and task-irrelevant items (e.g., Belopolsky, Theeuwes, & Kramer, 2005; Belopolsky et al., 2010; Burnham, Neely, Naginsky, & Thomas, 2010; Chua, 2013; Franconeri, Simons, & Junge, 2005; Schreij, Los, Theeuwes, Enns, & Olivers, 2014; Schreij, Owens, & Theeuwes, 2008; Schreij, Theeuwes, & Olivers, 2010a, 2010b; Theeuwes, 1994, 2004; Theeuwes, Kramer, Hahn, & Irwin, 1998; Theeuwes et al., 2000; Turatto & Galfano, 2001; Turatto et al., 2004; Yantis & Egeth, 1999). For example, Theeuwes (1994; see also, Theeuwes et al., 1998) had observers search for a color singleton target and observed capture by an onset singleton distractor. Additionally, using a task similar to that of Folk et al. (1992), Schreij et al. (2014) presented an onset distractor simultaneously with a display in which the target was defined by color and found the distractor elevated saccadic RTs to the target. In these and other studies, because the distractor was dissimilar to the target and irrelevant to the attentional settings, the attentional capture effects were considered stimulus driven.
One issue is each side of this debate must account for the other sides results. Specifically, those who claim salient stimuli capture attention in a stimulus-driven manner must explain why target features modulate attentional capture. Similarly, those who suggest top-down settings modulate the early selection of salient stimuli must accommodate results claiming to show task-irrelevant stimuli capturing attention. Part of the issue may be due to differences in tasks (visual search vs. spatial cuing), how targets are defined (singleton vs. conjunction), or the temporal synchrony between the cue and target (simultaneous vs. delayed). Nonetheless, different hypotheses have been proposed to account for the disparate findings, including attentional disengagement (e.g., Belopolsky et al., 2010; Theeuwes, 2010; Theeuwes et al., 2000), signal suppression (Sawaki & Luck, 2010), and attentional dwelling (e.g., Gaspelin, Ruthruff, & Lien, 2016).
Attentional disengagement
According to attentional disengagement, salient stimuli capture attention in a stimulus-driven manner, but the lack of cuing effects by task-irrelevant stimuli reflects a quick withdrawal of attention from the attention-capturing item (Belopolsky et al., 2010; Theeuwes, 2010; Theeuwes et al., 2000). Theeuwes et al. (2000) found that during search for a shape singleton target, a color singleton distractor delayed responding when the distractor appeared 50 ms or 100 ms prior to the target, but not when it appeared between 150 ms and 300 ms prior to the target (see also, Lamy & Egeth, 2003; Theeuwes & Godijn, 2002). This suggested attention was captured by the distractor, but quickly withdrew from the distractor, thereby nullifying any observable capture effect. Theeuwes et al.’s (2000) results provide an explanation for why spatial cuing studies, which typically use cue-target stimulus-onset asynchronies (SOAs) of about 150 ms, fail to observe cuing effects by target-irrelevant cues. However, using a variant of Folk et al.’s (1992) task, Chen and Mordkoff (2007) found target-irrelevant cues did not elicit cuing effects at 35-ms cue-target SOAs, which should be short enough to detect stimulus-driven capture. Although this goes against disengagement, other studies support Theeuwes et al.’s (2000) account.
For example, using a cuing task similar to Folk et al.’s (1992) and combined with a go/no-go task, Belopolsky et al. (2010), Experiment 4 found target-relevant cues appearing 150 ms prior to a target elicited positive cuing effects, consistent with contingent orienting, but target-irrelevant cues elicited negative cuing effects, that is, responding was faster following invalid cues. Belopolsky et al. (2010, p. 356), suggested “Suppression of the nonmatching cue location suggests that top-down attentional control could not prevent attentional capture by the cue but operated by rapid disengagement of attention from the cued location, followed by suppression of processing at that location...” (p.356, emphasis added). In other words, the target-irrelevant cue captured attention in a stimulus-driven manner, but attention withdrew from the cue and was then suppressed from returning.
Signal suppression
Sawaki and Luck (2010) developed a signal-suppression hypothesis, which proposes salient stimuli generate an “attend-to-me” signal, but attention shifts only toward salient and task-relevant stimuli, whereas attention is suppressed from selecting task-irrelevant stimuli. Sawaki and Luck (2010) examined evoked response potentials (ERPs) elicited by targets and salient distractors containing target-relevant or target-irrelevant features. Both targets and target-relevant distractors elicited an N2pc, while salient and target-irrelevant distractors did not. However, target-irrelevant distractors did elicit distractor positivity (Pd), an ERP marker of suppression of – but not capture by – a distracting object. The Pd component occurs in about the same timeframe and at the same recording sites as the N2pc, but is the increased positivity contralateral to an ignored or suppressed distractor (Burra & Kerzel, 2013; Hickey, Di Lollo, & McDonald, 2009). Indeed, Gaspar and McDonald (2014) found salient distractors can elevate RTs in a visual search task, without eliciting a lateralized N2pc to the distractor, but did elicit a lateralized Pd.
Signal suppression was supported by Gaspelin, Leonard, and Luck (2015) and by Gaspelin and Luck (2018), who observed impaired identification of probes that appeared near salient, but target-irrelevant distractors. Specifically, probe identification accuracy was lower for letter probes near a salient singleton compared to probes appearing near a non-singleton. Critically, Gaspelin, and Luck (2018) demonstrated this suppression effect depended on foreknowledge of the upcoming distractor color; hence, observers may suppress a feature, rather than the “attend-to me” signal. It is important to note that studies showing evidence of suppression typically use salient distractors that never appeared in a target’s location (see, however, Belopolsky et al., 2010); hence, it is not clear whether suppression occurs in cuing tasks where a cue may appear in the target’s location.
Attentional dwelling
Gaspelin et al. (2016) suggested that onsets capture attention in a stimulus-driven manner, but search difficulty moderates whether cuing effects are observed when the onset is irrelevant to the attentional set.Footnote 1 The dwelling hypothesis assumes attention shifts toward abrupt onsets and remains there until a nontarget item appears, providing a reason to reorient attention. On invalid trials, attention remains on an invalidly cued nontarget if it resembles the target (e.g., a nontarget letter “A” in a letter discrimination task between “E” and “H”). Once that nontarget is rejected, search of other nontargets occurs, which lengthens the responses and produces a cuing effect. In contrast, if the invalidly cued nontarget is dissimilar to the target (e.g., a green nontarget in a search for a red target), the nontarget is quickly rejected and reoriented toward the target, thereby nullifying the cuing effect. Hence, how long attention “dwells” at an invalidly cued location determines whether a cuing effect by an irrelevant onset is observed, and “dwelling” depends on how difficult it is to distinguish targets from nontargets – what Gaspelin et al. (2016) called “easy” and “difficult” searches. Gaspelin et al. (2016) observed cuing effects by abrupt onsets when target search was difficult (e.g., letter search), but not when search was easy (e.g., color search), suggesting attention dwelled on a featurally similar nontarget during difficult search. Importantly, the disengagement and dwelling hypotheses predict salient stimuli capture attention, but differ with respect to when and how attention reorients away from the attention-capturing item. For disengagement, attention reorients soon after being captured and before the target appears; whereas dwelling assumed attention reorients only after the target search display appears and the search task is easy; that is, attention “lingers until the target display appears.” (p. 1107)
Determining the locus of attention after cue appearance
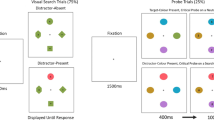
A question is where attention is focused after the appearance of a salient cue. Gaspelin et al. (2015) developed a capture-probe paradigm to provide an independent measure of the spread and focus of attention following the onset of a display (see Kim & Cave, 1999, for a similar task.) In this paradigm (Fig. 1) frequent search trials are intermixed with infrequent letter probe trials. On search trials, subjects report the identity of target, which in in the present study was whether a singleton was slanted left or right (\ or /). On probe trials, the cue display is presented just as on search trials, but briefly flashed and then masked letters appear at the potential target locations, and subjects recall as many letters as possible. Because probe trials proceed exactly as search trials, one can determine the focus of attention following cue onset by examining which letters are recalled. If a cue captured attention, a greater proportion of cued probe letters should be recalled than uncued probe letters.
The present study mixed letter probe trials with search trials to evaluate where attention was after the appearance of target-relevant and target-irrelevant cues. In the search task, subjects reported the identity of a color singleton or onset singleton that appeared after a briefly presented red cue or onset cue. On probe trials, a different and randomly chosen letter appeared in each of the potential target locations, followed by a response display that served as a mask. The probe display appeared just after the cue’s appearance; hence, the focus of attention in the cue display could be determined based on which letters were identified. Specifically, if the cue captured attention to its location, more cued probes should be identified than uncued probes; however, if the cue did not capture attention, cued and uncued probes should be identified equally. Importantly, probe identification performance yields a measure of attention within the cue display that is independent of search target processing (i.e., a search target is not presented), and is a more early processing or perceptual measure of where visual attention is focused. As such, probe identification should be a more sensitive measure of the possible stimulus-driven capture of attention than RTs in the search task.
Recently, Huffman, Antinucci, and Pratt (2018) reported two experiments using similar procedures to those used here. In a color singleton search task (Experiment 1), they found cuing effects in RTs were larger for color cues than onset cues, but probe identification cuing effects were equivalent. In an onset search task (Experiment 2), they found only onset cues produced cuing effects in RTs and probe identification. Though the procedures and results were like the present study, Huffman et al. (2018) presented letter probes at the same cue-target SOA as the stimuli in the search task (150 ms) in order to examine the utility of the letter probe task in cuing studies, and to examine sequential dependencies in attentional capture. In contrast, in the present study the letter probes appeared 50 ms after cue onset to examine where attention was focused after the appearance of the cue but before the appearance of the target. As such, the predictions regarding stimulus-driven capture may not be applicable to Huffman et al. (2018), because their longer cue-target SOA may obscure potential stimulus-driven effects.
Experiment 1
Experiment 1 combined a probe identification task with a spatial cuing task. On a majority of trials, subjects completed a cuing task similar to Folk et al. (1992). On a minority of trials, the cuing task was combined with the probe task, during which a briefly presented letter display appeared after the appearance of the cue. Performance on the probe task was used to evaluate where attention was located after the appearance of the cue.
Methods
Subjects
Sample size was based on an estimation by G*Power (Faul, Erdfelder, Buchner & Lang, 2007). Typically, contingent capture effects in the literature and our lab have been obtained with ns anywhere from 12 to 36; however, the primary interest in this study was differences in cued and uncued probe identification accuracies. As such, effect size for that difference was based on attentional capture effect in probe identification accuracy seen in Experiment 1 of Gaspelin et al. (2015), which was estimated to be dz = 0.7879. Assuming a priori Power (1 – β) of .95 and α = .05, the minimum sample size was n = 23. Increases from this number in any experiment resulted from more students than needed signing up for the study.
Twenty-three undergraduate students from The University of Scranton participated (19 females, 22 right-handed) and received credit toward a research requirement. Subjects ranged in age from 18 to 23 years (M = 18.61, SD = 1.16) and all reported normal or corrected-to-normal vision. All subjects passed an Ishihara color blindness test.
Apparatus
Experiments were programmed and presented using E-Prime software (v. 2.0.10242) on a Dell 755 computer with a Pentium Core 2 Duo processor (2.33 GHz; 1.96 GB RAM). Displays were presented on a Dell E178Fpv monitor (60 Hz; 1,024 × 768) that was height-adjusted to each subject sitting approximately 60 cm from the monitor. Subjects responded on a five-button serial response box. An OptiCAL RS-232 photometer (Cambridge Research Systems) was used to take luminance readings from a 1° × 1° color patch on a black background presented on the screen.
Stimuli
See Fig. 1. All displays were presented on a black background (0.16 cd/m2, RGB: 0, 0, 0) and included a square placeholder (1.53° × 1.53°) with a white (25.77 cd/m2, RGB: 255, 255, 255) two-pixel border at each of four potential target locations. Each target location placeholder was centered 4.48° from a white central fixation cross (+). The spatial cue consisted of filled circles (diameter 0.24°) positioned 0.2° from the edges of the placeholders and 1.9° from the center. Cue displays included an onset cue or a red cue. For the onset cue, four white filled circles flanked the edges of one placeholder. For the red cue, four filled circles were presented around each placeholder; one set of dots was red (12.91 cd/m2, RGB: 255, 0, 0) and the other three sets were white. Target displays included an onset target or red target. For onset targets, a single white / or \ in 24-pt sans serif font (0.5° × 0.5°) appeared and was centered in one of the placeholders. For red targets, a red / or \ appeared in one of the placeholders simultaneously with white / or \ in the other placeholders, such that two / and two \ appeared in each display.
The letter probe display consisted of four white letters in sans serif font (0.5° × 0.5°) that were randomly chosen without replacement from the English alphabet. The probe response display contained all 26 letters of the English alphabet in order and an “OK” key. Subjects used the computer mouse to select between zero and four letters, and clicked “OK” to continue. The probe response display served as a mask for the probe display.
Design
The design was a 2 (Target Type: Onset vs. Red) × 2 (Cue Type: Onset vs. Red) × 2 (Cue Location: Valid, Invalid) mixed factorial with target type manipulated between subjects. The peripheral cue was valid when the cue coincided with the target’s location and invalid when the cue did not coincide with the target’s location. Cue type was blocked and counterbalanced within subjects. Cue location was randomized within blocks.
Procedure
After signing a consent form, all subjects were administered an Ishihara color blindness test. The computer monitor was then adjusted so a fixation point was at the subject’s eye level. Subjects received oral instructions that were also presented on the computer monitor. Subjects in the onset target group were told to locate and identify the single abruptly-onset target (/ or\), and subjects in the red target group were told to locate and identify the one red target among three white nontargets (/ or \). Subjects were asked to identify the target as quickly and with as few errors as possible. They were further informed whether a color cue or onset cue would precede the target, with cue type being blocked and counterbalanced for order.
Each session began with a block of 16 practice trials that included only the target display to familiarize subjects with the task. For each cue type, subjects completed a block of 32 practice trials without the letter probe, and a block of 40 practice trials with the letter probe. They then completed six blocks of 40 trials each with self-paced breaks between blocks. Thus, each subject completed 480 data collection trials (240 for each target type), which included 20% letter probe trials and 80% search trials. (Total number of trials, including all practice trials, was 640.)
Each trial began with a 500-ms fixation display followed by a warning display without the fixation point for 100 ms. The fixation displayed reappeared for 400–900 ms (randomly chosen in steps of 100 ms) and was followed by the spatial cue display for 50 ms. For the regular trial procedure, the target display appeared 100 ms after the offset of the cue display and remained visible for 3,000 ms, or until the subject responded. Subjects identified whether the target was a “/” or “\”. Incorrect responses or responses longer than 2,000 ms were indicated by a 500-ms, 500-Hz tone. The next trial began 1,000 ms after a subject responded or after the offset of the tone. For the probe trial procedure, the probe display appeared immediately after the offset of the cue display for 100 ms. Following, the probe response display and mask remained visible until the subject responded. The next trial began after the subject clicked the “OK” key. At the end of each designated block, subjects were encouraged to rest their eyes for as long as needed before continuing.
Results
For the search task, error trials and trials with RTs faster than 150 ms or greater than 2,000 ms were removed. Trimming resulted in the exclusion of 2.8% of the trials (2.6% due to error). Each subject’s MRT was computed for each cell in the cue type by cue validity. Arithmetic means appear in Table 1 and are plotted in Fig. 2. For the letter probe task, on each trial the number of correctly identified letters was determined. Next, the proportion of correctly identified cued and uncued probe letters was calculated for each cell in the design. Mean proportions of correctly identified cued and uncued probes appear in Table 1 and are plotted in Fig. 3.
Response times
Each subject's MRT was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) ANOVA with repeated measures on the factors cue type and cue validity. The ANOVA revealed a main effect of cue validity [F(1,21) = 31.12, MSE = 744.79, p < .001, Cohen’s f = 1.40] due to faster responding following valid cues (M = 554 [546,562]) than invalid cues (M = 591 [582,599]). The cue validity by target interaction approached significance [F(1,21) = 3.64, MSE = 744.79, p = .07, f = .42]. Most importantly, the three-way interaction was significant [F(1,21) = 24.48, MSE = 540.08, p < .001, f =1.08]. No other effects were significant [Fs < 1, ps > .744]. As seen in Table 1 and in Fig. 2, the three-way interaction reflects a pattern of contingent attentional capture. In the red target group, the cuing effect was significant for red cues [t(10) = 5.85, SE = 11.60, p < .001, d = 1.02], and also but unexpectedly for onset cues [t(10) = 3.68, SE = 7.33, p = .004, d = .43]. In the onset target group, the cuing effect was significant for onset cues [t(11) = 4.16, SE = 12.81, p = .002, d = .53], but not red cues [t < 1].
Errors
Each subject's percentage of errors was entered into a similar ANOVA, but no effects approached significance [Fs < 2.43, ps > .134].
Probe identification
The proportion of correctly identified cued and uncued letters for each subject was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of correctly identified cued letters (M = .48) than uncued letters [M = .32; F(1,21) = 22.81, MSE = 0.024, p < .001, Cohen’s f = 1.04]. Interactions were observed between cue type and target type [F(1,21) = 12.41, MSE = 0.003, p = .002, Cohen’s f = .88] and target type and letter probe [F(1,21) = 11.22, MSE = 0.024, p = .003, Cohen’s f = .73]. Most importantly, the three-way interaction was significant [F(1,21) = 48.95, MSE = 0.006, p < .001, Cohen’s f = 1.29]. No other effects approached significance [Fs < 1.78, ps > .196]. As seen in Table 1 and Fig. 3, the interaction reflects a pattern similar to the RT analyses, that is, probe identification greater for letters cued by a target-relevant cue. In the red target group, probe enhancement was significant for red cues [t(10) = 5.93, SE = 0.064, p < .001, d = 2.48], and nearly for onset cues [t(10) = 1.92, SE = 0.075, p = .084, d = .88]. In the onset target group, the enhancement was significant for onset cues [t(11) = 5.03, SE = 0.031, p < .001, d = 1.88], and was unexpectedly and significantly negative for red cues [t(11) = -2.88, SE = 0.023, p = .015, d = -.86].
Discussion
The results showed patterns consistent with target features modulating the cuing effects in RTs to the search targets, and enhancement in probe identification. Specifically, cuing effects in RTs and probe enhancement effects were greater for target-relevant cues than the target-irrelevant cues. Thus, the probe task was adapted to a cuing task, as in Huffman et al. (2018).
The patterns in Figs. 3 and 4 show the target feature modulated both the RT cuing effects and probe enhancement results, but also revealed possible evidence for stimulus-driven capture. Specifically, the positive probe enhancement effect for onset cues in the red target group (.14), suggests the cue captured attention. Additionally, the negative effect for red cues in the onset target group (-.07) suggests the cue was suppressed. As such, Experiments 2–6 were conducted to examine the influence of attentional settings on the probe enhancement effects observed in Experiment 1.
Experiment 2
According to attentional disengagement, salient stimuli attract attention, but attention rapidly moves away from irrelevant stimuli once top-down control takes over (Theeuwes et al., 2001). In Experiment 1, the SOA was 50 ms, which although within the range used by Theeuwes et al. (2001), could have provided time for attention to orient away from the cued location and nullify probe enhancement. In Experiment 2, the cue-probe SOA varied, and if the target-irrelevant cues captured attention which was then rapidly disengaged, probe enhancement effects should decrease as SOA increased for the target-irrelevant cues.
Methods
Unless otherwise noted, all methods were the same as Experiment 1.
Subjects
Twenty-four undergraduates participated (19 females, 21 right-handed). Subjects ranged in age from 18 to 21 years (M = 18.63 SD = 0.77) and reported normal or corrected-to-normal vision.
Procedure
The search task was the same as Experiment 1. In the probe task, subjects received the same instruction as Experiment 1; however, the probe display appeared 17 ms, 34 ms, or 51 ms after the onset of the cue display. As in Experiment 1, the cue display remained presented for 50 ms and the probe display remained present for 100 ms. To accommodate the three cue-probe SOAs, the number of trials was increased for each cue type. Subjects completed six blocks of 44 trials for a total of 632 trials, with 27.3% letter probe trials and 72.7% search trials.
Results
The same trimming from Experiment 1 was used and resulted in the exclusion of 3.1% of the trials (2.8% due to error). Each subject’s MRT was computed for each cell in the cue type by cue validity design for each target group and arithmetic means appear in Table 1 and are plotted in Fig. 2. For the probe task, the proportion of correctly identified cued and uncued letters was determined and the mean proportions appear in Table 1 and are plotted in Fig. 4.
Response times
Each subject's MRT was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) ANOVA with repeated measures on the factors cue type and cue validity. The ANOVA revealed a main effect of cue validity [F(1,22) = 56.98, MSE = 690.17, p < .001, Cohen’s f = 1.61] due to faster responding following valid cues (M = 577 [569,584]) than invalid cues (M = 617 [609,625]). Responding was faster to onset targets (M = 556 [504,608]) compared to red targets (M = 638 [586,690]), [F(1,22) = 5.36, MSE = 30151.73, p = .03, f = .49]. The cue validity by cue type interaction was significant [F(1,22) = 5.01, MSE = 357.23, p = .036, f = .48]. Most importantly, the three-way interaction was significant [F(1,22) = 25.48, MSE = 357.23, p < .001, f =1.08]. No other effects were significant [Fs < 2.14, ps > .158]. As seen in Table 1 and in Fig. 2, the three-way interaction reflects a pattern of contingent attentional capture; however, as in Experiment 1, the onset cue did elicit a cuing effect in the red target group. In the red target group, the cuing effect was significant for red cues [t(11) = 4.73, SE = 10.86, p = .001, d = .43], and unexpectedly for onset cues [t(11) = 3.13, SE = 9.50, p = .01, d = .25]. In the onset target group, the cuing effect was significant for onset cues [t(11) = 8.49, SE = 8.11, p < .001, d = 1.51], but not for red cues [t(11) = 1.42, SE = 8.67, p = .184, d = .21 ].
Errors
Each subject's percent errors was entered into a similar ANOVA, which revealed only a nearly significant three-way interaction [F(1,22) = 3.06, MSE = 0.001, p = .094, Cohen’s f = .37]. No other effects approached significance [Fs < 1.38, ps > .251].
Probe identification
The proportion of correctly identified cued and uncued probe letters for each subject was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 3 (SOA: 17, 34, 51) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of identified cued letters (M = .45) than uncued letters [M = .34; F(1,22) = 37.05, MSE = 0.023, p < .001, Cohen’s f = 1.30]. The difference in probe identification between onset cues (M = .41) and red cues (M = .38) was significant [F(1,22) = 10.25, MSE = 0.005, p = .004, Cohen’s f = .68]. Interactions were observed between cue type and target type [F(1,22) = 15.489, MSE = 0.005, p = .001, Cohen’s f = .84], and between cue type and letter probe [F(1,22) = 22.41, MSE = 0.008, p < .001, Cohen’s f = 1.01]. Most importantly, the three-way interaction was statistically significant [F(1,22) = 48.72, MSE = 0.008, p < .001, Cohen’s f = 1.49]. No other effects were statistically significant, including all interactions with SOA [Fs < 2.73, ps > .076]. As seen in Table 1 and Fig. 3, the interaction reflects a similar pattern to the RT analyses. The pattern of contingent capture was unaffected by SOA; however, as in Experiment 1, more letters were identified when cued by the onset in the red target group. In the red target group, the enhancement effect was significant for red cues [t(11) = 3.96, SE = 0.041, p = .002, d = 1.12], and surprisingly for onset cues [t(11) = 3.97, SE = 0.029, p = .002, d = .90]. In the onset target group, the enhancement effect was significant for onset cues [t(11) = 7.87, SE = 0.026, p < .001, d = 2.01], and significantly negative for red cues [t(11) = -2.62, SE = 0.017, p = .024, d = -.48].
Discussion
The results largely replicated Experiment 1 in RTs and probe identification, including the positive probe enhancement effects for onset cues in the red target group. Experiment 2 failed to show a decrease in probe enhancement as a function of cue-probe SOA (Fig. 4), which fails to support disengagement. Nonetheless, the probe enhancement effect and RT cuing effects for onsets in the red target group, along with the small negative probe effect for red cues in the onset target group are somewhat at odds and unexpected. These were examined in Experiments 3–6.
Experiment 3
In Experiment 3, red cue and onset cue trials were intermixed within blocks. This was done to examine whether the blocked cue design was a factor in producing the positive effect for onset cues and the small negative effect for red cues.
Methods
Unless otherwise noted, all methods were the same as Experiment 1.
Subjects
Thirty students participated (24 females, 25 right-handed). Subjects ranged in age from 18 to 21 years (M = 18.67, SD = 0.88) and all reported normal or corrected-to-normal vision.
Procedure
All procedures were the same as Experiment 1, except cue type was randomized within blocks. To accommodate for this change, subjects completed six blocks of 80 trials each for a total of 592, with 20% letter probe trials and 80% were search trials.
Results
The trimming procedure in Experiments 1 and 2 was used and resulted in the exclusion of 2.5% of the trials (nearly all errors). Each subject’s MRT was computed for each cell in the cue type by cue validity design. Arithmetic means appear in Table 1 and are plotted in Fig. 2. For the probe task, the proportion of correctly identified cued and uncued letters was determined and the mean proportions appear in Table 1 and are plotted in Fig. 3.
Response times
Each subject's MRT was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) ANOVA with repeated measures on the factors cue type and cue validity. The ANOVA revealed a main effect of cue validity [F(1,28) = 160.18, MSE = 262.91, p < .001, Cohen’s f = 2.39] due to faster responding following valid cues (M = 548 [544,552] than invalid cues (M = 586 [581,590]). The main effect of cue type was significant [F(1,28) = 8.23, MSE = 432.156, p = .008, Cohen’s f = .54]. The only other effect to reach significance was the three-way interaction [F(1,28) = 107.34, MSE = 234.81, p < .001, Cohen’s f = 1.96]. No other effects were significant [Fs < 2.72, ps > .11]. As seen in Table 1 and Fig. 2, the interaction reflects a pattern of contingent attentional capture; however, as in Experiments 1 and 2, the onset cue did elicit a cuing effect in the red target group. In the red target group, the cuing effect was significant for red cues [t(14) = 9.22, SE = 7.65, p < .001, d = .69], and onset cues [t(14) = 3.16, SE = 4.33, p = .007, d = .13]. In the onset target group, the cuing effect was significant for onset cues [t(14) = 10.40, SE = 6.00, p < .001, d = .60], but not red cues [t < 1].
Errors
A similar analysis of the percent errors revealed a significant effect of cue type [F(1,28) = 8.92, MSE = 0.001, p = .006, Cohen’s f = .56] due to fewer errors following onset cues (1.8%) than red cues (3.0%). Both the cue type by cue validity interaction [F(1,28) = 3.58, MSE = 0.001, p = .069, Cohen’s f = .36] and the three-way interaction [F(1,28) = 3.05, MSE = 0.001, p = .092, Cohen’s f = .33] approached significance. No other effects approached significance [Fs < 1.38, ps > .25].
Probe identification
The proportion of identified cued and uncued letters for each subject was entered into a 2 (Target: Color vs. Onset) × 2 (Cue Type: Color vs. Onset) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of correctly identified cued letters (M = .46) than uncued letters [M = .37; F(1,28) = 50.96, MSE = 0.005, p < .001, Cohen’s f = 1.35]. The effect of cue type was also significant [F(1,28) = 5.04, MSE = 0.002, p = .033, Cohen’s f = .42], as were interactions between cue type and target type [F(1,28) = 7.81, MSE = 0.002, p = .00953, Cohen’s f = .75], and target type and probe [F(1,28) = 9.28, MSE = 0.005, p = .005, Cohen’s f = .58]. Most importantly, the three-way interaction was significant [F(1,28) = 41.57, MSE = 0.004, p < .001, Cohen’s f = 1.22]. No other effects approached significance [Fs < 1, ps > .398]. As seen in Table 1 and in Fig. 3, and like in Experiments 1 and 2, there was increased probe identification to letters cued by the onset cue in the red target group. In the red target group, the enhancement effect was significant for red cues [t(14) = 5.84, SE = 0.033, p < .001, d = 1.22] and onset cues [t(14) = 3.20, SE = 0.021, p = .062, d = .46]. In the onset target group, the effect was significant for onset cues [t(14) = 6.26, SE = 0.021, p < .001, d = 1.24], and negative, but not statistically significant, for red cues [t(14) = -1.65, SE = 0.017, p = .121, d = -.26].
Discussion
The results largely replicated Experiments 1 and 2: the target feature modulated the RT cuing effects and probe enhancement effects, but a positive probe enhancement effect and RT cuing effect for onset cues was found in the red target group, along with a small negative probe effect for red cues in the onset group. Because the former has been consistently smaller than the effect produced by red cues in the red groups, this may reflect intermittent capture by onsets on a minority of trials. Additionally, though not statistically significant in Experiment 3 (p = .12), the negative probe effect by red cues is consistent with observers suppressing that location. This was examined in Experiments 4 and 5
Experiment 4
In Experiments 1–3, the negative probe enhancement effect suggests the location of the red cue was ignored, and Experiments 4 and 5 were conducted to explore whether subjects suppressed the target-irrelevant red cue. Experiment 4 was similar to Folk and Remington (1998), where subjects identified red or green targets preceded by red cues, green cues, or no cue. Folk and Remington (1998) observed contingent capture, but also found responding was slower following target-irrelevant cues than when no cue appeared; an effect they referred to as perceptual filtering. Because the no-cue condition provides a baseline and attention should not orient toward the periphery; if subjects suppressed the target-irrelevant cue, cued probe identification should be lower following target-irrelevant cues than when no cue appeared.
Methods
Unless otherwise noted, all methods were the same as Experiment 1.
Subjects
Twenty-three students participated (20 females, 22 right-handed). Subjects ranged in age from 18 to 21 years (M = 18.61, SD = 0.84) and all reported normal or corrected-to-normal vision.
Procedure
Procedures were similar to Experiment 1, with the following exceptions. First, onset cues and onset targets were replaced with green cues and green targets. The green cue and green target had the same characteristics as the red cue and red targets, respectively, with the only difference being the color of the cue or target was green (13.74 cd/m2). Second, a cue-absent block was included in addition to the red cue and green cue blocks. For cue absent trials, the cue display included sets of four white dots around each of the potential target locations. For each cue type, subjects completed five blocks of 40 trials each, with 20% letter probe trials and 80% were search trials.
Results
One subject’s data were excluded from analyses due to computer error. The trimming procedure in Experiments 1–3 resulted in the exclusion of 3.8% of the trials (3.3% errors). Each subject’s MRT was computed for each cell in the cue type by cue validity design for each target group and arithmetic means appear in Table 1 and are plotted in Fig. 2. For the probe task, the proportion of identified cued and uncued letters was determined and the mean proportions appear in Table 1 and are plotted in Fig. 3.
Response times
To examine spatial capture of attention, each subject's MRT was entered into a 2 (Target: Red vs. Green) × 2 (Cue Type: Red vs. Green) × 2 (Cue Validity: Valid vs. Invalid) ANOVA with repeated measures on the factors cue type and cue validity. The ANOVA revealed a main effect of cue validity [F(1,20) = 31.57, MSE = 920.95, p < .001, Cohen’s f = 1.25] due to faster responding following valid cues (M = 583 [573,592] than invalid cues (M = 619 [610,629]). The only other effect to reach significance was the three-way interaction [F(1,20) = 24.49, MSE = 520.76, p < .001, Cohen’s f = 1.10]. No other effects were significant [Fs < 1.50, ps > .236]. As seen in Table 1 and in Fig. 2, the interaction reflects a pattern of contingent capture; however, the green cue did elicit a small positive cuing effect in the red target group. In the red target group, the cuing effect was significant for red cues [t(11) = 5.26, SE = 11.56, p < .001, d = .85], and nearly for green cues [t(11) = 2.17, SE = 11.26, p = .053, d = .31]. In the green target group, the cuing effect was significant for green cues [t(9) = 4.12, SE = 14.66, p = .003, d = .77], but not red cues [t<1].
To examine suppression of target-irrelevant cue, a distractor present condition was created by combining the validly cued RTs following target-irrelevant cues in each group (red valid cues for green targets; green valid cues for red targets). Responding in the distractor present condition (M = 596 [561,630]) was faster but not significantly different from the cue absent condition [(M = 611 [569,636]); t(21) = 1.08, SE = 14.23, p = .294, d = .18].
Errors
The percent errors were first entered into a 2 (Target: Red vs. Green) × 2 (Cue Type: Red vs. Green) × 2 (Cue Validity: Valid vs. Invalid) ANOVA, which failed to yield any statistically significant effects [Fs < 2.12, ps > .161].
Probe identification
The proportion of correctly identified cued and uncued probe letters for each subject were first entered into a 2 (Target: Red vs. Green) × 2 (Cue Type: Red vs. Green) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of identified cued letters (M = .50) and uncued letters [M = .31; F(1,20) = 37.13, MSE = 0.022, p < .001, Cohen’s f = 1.36]. The only other effect to reach significance was the three-way interaction [F(1,20) = 31.77, MSE = 0.006, p < .001, Cohen’s f = 1.26]. No other effects were significant [Fs < 1.71, ps > .206]. As seen in Table 1 and in Fig. 3, the interaction reflects contingent capture; however, the green cue did elicit a small positive cuing effect in the red target group. In the red target group, the enhancement effect was significant for red cues [t(11) = 4.04, SE = 0.065, p = .002, d = 1.64] and for green cues [t(11) = 2.70, SE = 0.040, p = .021, d = .79]. In the green target group, the enhancement effect was significant for green cues [t(9) = 6.01, SE = 0.052, p < .001, d = 2.54], and for red cues [t(9) = 2.53, SE = 0.033, p = .032, d = .86].
To examine the presence of suppression, the distractor present condition was created by combining the proportions of identified cued probes for the target-irrelevant cue in each group. (Uncued probes responses were not used, because no suppression should be placed at the uncued location.) The proportion of correctly identified letters in the distractor present condition (M = .45) was marginally greater – not less – than the proportion of letters identified in the distractor absent condition (M = .40; [t(21) = 2.00, SE = 0.021, p = .059, d = .34].
Discussion
Experiment 4 showed that the target features moderated the cuing effects and probe enhancement effects, but failed to show cued probe identification was lower following target-irrelevant cues than when the cue was absent. Although inconsistent with observers suppressing the target-irrelevant cue, Experiment 5 provided a second test of suppression by using the onset cues and targets from Experiments 1–3.
Experiment 5
Experiment 5 was conducted to examine whether evidence for suppression could be obtained with onset cues and targets. The onset cue and onset targets from Experiments 1–3 were used along with a cue absent condition. If observers suppressed the target-irrelevant cues, cued probe identification should be lower following target-irrelevant cues than no cue.
Methods
Unless otherwise noted, all methods were the same as Experiments 1 and 4.
Subjects
Twenty-five students participated (17 females, all right-handed). Subjects ranged in age from 18 to 21 years (M = 18.56, SD = 0.77) and all reported normal or corrected-to-normal vision.
Procedure
All procedures were the same as Experiments 1 and 4. Experiment 5 used the same red cues and onset cues and red targets and onset targets as Experiment 1; and like Experiment 4 also included a cue-absent block for both target groups.
Results
A computer error prevented the cue-absent block from being presented for five subjects in the red target group. Those subjects’ data were not included in the analyses, and one additional subject was excluded due to low accuracy. The trimming in Experiments 1–4 resulted in the exclusion of 3.4% of the trials (3.1% errors). Each subject’s MRT was computed for each cell in the cue type by cue validity design for each target group. Arithmetic means appear in Table 1 and are plotted in Fig. 2. The proportion of correctly identified cued and uncued probe letters was determined and the mean proportions appear in Table 1 and are plotted in Fig. 3.
Response times
To examine spatial capture of attention, each subject's MRT was entered into a 2 (Target: Red vs. Onset) × 2 (Cue Type: Red vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) ANOVA with repeated measures on the factors cue type and cue validity. The ANOVA revealed a main effect of cue validity [F(1,17) = 43.48, MSE = 611.403, p < .001, Cohen’s f = 1.60] due to faster responding following valid cues (M = 552 [543,560]) than invalid cues (M = 591 [582,599]). The only other effect to reach statistical significance was the three-way interaction [F(1,17) = 32.16, MSE = 610.57, p < .001, Cohen’s f = 1.37]. No other effects were significant [Fs < 1.11, ps > .306]. As seen in Table 1 and in Fig. 2, the interaction reflects contingent attentional capture. In the red target group, the cuing effect was significant for red cues [t(6) = 5.44, SE = 12.61, p = .002, d = .79], but not onset cues [t < 1]. In the onset target group, the cuing effect was significant for onset cues [t(11) = 6.47, SE = 11.68, p < .001, d = .91], but not red cues [t<1].
To examine signal suppression, a distractor present condition was created in the same way as in Experiment 4. The distractor present condition (M = 560 [521,600]) was not significantly different from the cue absent condition [(M = 565 [520,609]); t(18) = 0.27, SE = 15.69, p = .788, d = .05].
Errors
The percent errors were first entered into a 2 (Target: Red vs. Onset) × 2 (Cue Type: Red vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) ANOVA. The three-way interaction was nearly statistically significant [F(1,17) = 4.40, MSE = .001, p = .051, Cohen’s f = .51], but no other effects approached statistical significance [Fs < 1.32, ps > .266].
Probe identification
The proportion of correctly identified cued and uncued probe letters for each subject were first entered into a 2 (Target: Red vs. Onset) × 2 (Cue Type: Red vs. Onset) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of cued letters (M = .47) and uncued letters [M = .36; F(1,17) = 40.52, MSE = 0.005, p < .001, Cohen’s f = 1.54]. This effect interacted with target type [F(1,17) = 12.92, MSE = 0.005, p = .002, Cohen’s f = .87], but more importantly the three-way interaction was also statistically significant [F(1,17) = 36.27, MSE = 0.004, p < .001, Cohen’s f = 1.46]. No other effects approached significance [Fs < 2.52, ps > .131]. As seen in Table 1 and in Fig. 3, the interaction reflects contingent capture. In the red target group, the enhancement effect was significant for red cues [t(6) = 5.35, SE = 0.049 p = .002, d = 2.25], and onset cues [t(6) = 2.65, SE = 0.028 p = .038, d = .28]. In the onset target group, the cuing effect was significant for onset cues [t(11) = 5.35, SE = 0.027, p < .001, d = 1.10], and negative, but not statistically significant for red cues [t(11) = -1.94, SE = 0.026 p = .078, d = -.42].
To examine suppression, the distractor present condition was created by combining the proportions of correctly identified cued probes for the target-irrelevant cue in each target group. The proportion of correctly identified probes in the distractor present condition (M = .40) was not different from the proportion of probes identified in the distractor absent condition (M = .40; [t(17) = .03, SE = 0.024, p = .979, d = .01].
Discussion
Experiment 5 largely replicated Experiment 4: target features moderated the cuing effects and probe enhancement effects, but cued probe identification was no different from the cue absent condition. It is noteworthy the positive cuing effect for onsets in the red target group was absent in Experiment 5; however the probe enhancement effect was present, though smaller than the effect for red cues.
Experiment 6
The purpose of Experiment 6 was to establish a strong template for the target, which should eliminate the onset cuing effect in RTs and probe enhancement in Experiments 1–5. Subjects identified the orientation of a red target that was uniquely colored among three other nontargets. By presenting the target and each nontarget in a different color, observers would need to adopt a feature search for the red target.
Methods
Unless otherwise noted, all methods were the same as in Experiment 3.
Subjects
Because only one target type was used in Experiment 6, a sample size of only n = 12 was needed. Thirteen students participated (10 females, 11 right-handed). Subjects ranged in age from 18 to 24 years (M = 19.38, SD = 1.66) and all reported normal or corrected-to-normal vision.
Stimuli
Each target display included two left-slanted (\) and two right-slanted (/) lines. One item (target) was red) and each nontarget was randomly chosen to be teal (0,108,100), magenta (183,0,183), orange (170,87,0), or yellow (200,200,0). Hence, the only feature that signaled the target was its color. The cue displays were the same as in Experiments 1, 2, 3, and 5, with a red cue or onset cue presented on each trial. Cue type was randomized within blocks (as in Experiment 3), but each cue appeared equally often a cross trials. The probe displays and task was identical to Experiments 1–5.
Procedure
Each trial proceeded as in Experiment 3. Subjects completed a block of 16 practice trials without the cue, 16 practice trials with the cue, and 40 combined task practice trials, followed by six blocks of 80 trials each. Within each block, the probe task appeared on a random 20% of the trials. Cue type (onset, red), target type (\, /), and task (probe identification, search) were randomized within blocks.
Results
The same trimming used in Experiments 1–5 resulted in the exclusion of 3.3% of the trials (3.0% errors). Each subject’s MRT was computed for each cell in the cue type by cue validity design. Arithmetic means appear in Table 1 and are plotted in Fig. 2. The proportion of correctly identified cued and uncued probe letters was determined and the mean proportions appear in Table 1 and are plotted in Fig. 3.
Response times
Each subject's MRT was entered into a 2 (Cue Type: Red vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) repeated-measures ANOVA. The main effect of cue validity was significant [F(1,12) = 52.24, MSE = 309.02, p < .001, Cohen’s f = 2.08] due to faster responding following valid cues (M = 657 [650,665]) than invalid cues (M = 692 [685,700]). The main effect of cue type was also significant [F(1,12) = 10.45, MSE = 320.78, p = .007, Cohen’s f = .93] due to faster responding following red cues (M = 667 [659,674]) than onset cues (M = 683 [675,690]). Although the interaction did not reach statistical significance [F(1,12) = 3.41, MSE = 927.08, p = .089, Cohen’s f = .53], as seen in Table 1 and in Fig. 2, the cuing effect was significant for red cues [t(12) = 6.33, SE = 8.03, p < .001, d = .55], but was smaller and not significant for onset cues [t(12) = 1.75, SE = 11.22, p = .105, d = .20].
Errors
The percent errors were entered into a 2 (Cue Type: Red vs. Onset) × 2 (Cue Validity: Valid vs. Invalid) repeated-measures ANOVA; however, no effects were statistically significant [Fs < 2.32, ps > .153].
Probe identification
The proportion of correctly identified cued and uncued probe letters for each subject were entered into a 2 (Cue Type: Red vs. Onset) × 2 (Letter Probe: Cued vs. Uncued) ANOVA. The analysis revealed a difference in the proportion of cued letters (M = .40) and uncued letters [M = .29; F(1,12) = 45.16, MSE = 0.003, p < .001, Cohen’s f = 1.94]. More probes were identified following red cues (M = .36) than onset cues [M = .33; F(1,12) = 9.42, MSE = 0.001, p = .01, Cohen’s f = .89]. Importantly the interaction was statistically significant [F(1,12) = 9.27, MSE = 0.006, p = .01, Cohen’s f = .88]. As seen in Table 1 and in Fig. 3, the enhancement effect was significant for red cues [t(12) = 7.44, SE = 0.023 p < .001, d = 2.19], but smaller and not significant for onset cues [t(12) = 1.53, SE = 0.029 p = .153, d = .57].
Discussion
Experiment 6 encouraged observers to establish a strong target template by adopting a feature search for the one red item among several differently colored nontargets. The results revealed only the red cue elicited a significant RT cuing effect and probe enhancement effect. Thus, a ‘weak’ target template or intermittent lapses in attentional control may have allowed the onset cue to capture attention on some trials in Experiments 1–5. Indeed, that the onset cuing effect and enhancement effect were eliminated in Experiment 6 suggests those effects seen in Experiments 1–5 reflected variations in the establishment of a top-down set across trials; a point I address in the General discussion
Meta-analysis
Despite subtle differences in procedures, stimuli, and results across Experiments 1–6, the pattern was similar with the cuing effects for target-relevant cues being larger than for target-irrelevant cues. However, the cuing effect and probe enhancement effect for onset cues (and green cues in Experiment 4) was consistently positive in the red target group. Additionally, in several experiments, the probe enhancement effect for red cues was negative in the onset target group. Reasons are examined in the General discussion, but I first quantified and examined the size of cuing effects for target-relevant cues and target-irrelevant cues across all target types; that is, given the differences in cuing and probe enhancement effects across experiments, it was necessary to combine those effects to provide overall estimates of the effects elicited by target-relevant and by target-irrelevant cues.
Meta-analysis method
For each target in each experiment, the cuing effects (Table 1) for target-relevant cues and target-irrelevant cues were obtained for both RTs and probe identifications. Based on the equations recommended by Lipsey and Wilson (2001), unstandardized mean effect sizes (ESu)Footnote 2, standard errors (SEu)Footnote 3, and inverse variances (wu)Footnote 4 were calculated for the invalid/uncued and valid/cued conditions. Standardized effect sizes (Cohen’s d) were not used, because (a) the same measures were used across experiments, (b) sample sizes (per target group) were similar, and (c) the same participant pool was used. Mean cuing effects sizes (\( \overline{ES} \)) for the target-relevant and target-irrelevant cues were calculated by summing the unstandardized cuing effects (ESu) weighted by the inverse variances (wu) and then dividing by the summed weights. The standard error of the mean cuing effect size (\( {SE}_{\overline{ES}} \)) was calculated by taking the square root of the sum of the inverse variances (Lipsey & Wilson, 2001). Relevant statistics appear in Table 2.
Results
Figures 6 and 7 present forest plots of the cuing effects in RTs and probe identifications, respectively, arranged from smallest (bottom) to largest (top) based on the target-relevant cuing effects. Individual experiment/target combinations appear as circles and mean cuing effect sizes appear as diamonds. All data points include the 95% confidence interval (CI). For the individual target/experiment combinations, CIs were based on SEu and the critical t-Value for df = n – 1. The CI for the mean cuing effect sizes were based on \( {SE}_{\overline{ES}} \) and z = 1.96.
Examination of Figs. 6 and 7 indicates cuing effects by target-relevant cues were larger than target-irrelevant cues. This was true for the mean cuing effect in RTs (65.10 ms vs. 9.70 ms) and probe enhancement effect (0.1791 vs. 0.010). The mean RT cuing effect was above 0 ms for relevant cues [z = 24.07, \( {SE}_{\overline{ES}} \) = 2.70] and for irrelevant cues [z = 4.76, \( {SE}_{\overline{ES}} \) = 2.04], but as seen in the CIs, the effect for relevant cues was larger than irrelevant cues. For probe enhancement, the mean effect size was above 0 for relevant cues [z = 19.05, \( {SE}_{\overline{ES}} \) = 0.009], but not for irrelevant cues [z = 1.36, \( {SE}_{\overline{ES}} \) = 0.007], and as seen in the CIs, the effect for relevant cues was larger than for irrelevant cues.
To examine cuing effects for each cue/target combination from Experiments 1, 2, 3, 5, and 6, all of which used the same cue types and target types, mean effect sizes and standard errors in RTs and probe identifications were calculated. A plot of the cuing effects in RTs against probe identifications is presented in Fig. 7, where it can be seen the cuing effects for relevant cues (upper right) were larger than those for irrelevant cues (bottom left). Additionally, based on the CIs for target-irrelevant cues, cuing effects by onset cues for red targets indicate a benefit in RTs and probe identification; whereas cuing effects by red cues for onset targets indicate no benefit in RTs and slight suppression for probe identification.
Discussion
The results indicate target-relevant cues elicited larger cuing effects and probe enhancement effects than the target-irrelevant cues (Figs. 5, 6 and 7). Although the effect size was above zero for irrelevant cues in RTs (Fig. 5), this was not so for probe enhancement (Fig. 6). The probe enhancement effect size for target-irrelevant cues was not larger than zero, and the positive effect size in RTs for irrelevant cues (Fig. 5) was due solely to the positive cuing effect for onset cues in the red target groups (Fig. 7). Additionally, while the overall probe enhancement effect for target-irrelevant cues was not different than 0, the effect was above 0 for onset cues in the red target group, and below 0 for red cues in the onset target group. These observations from Fig. 7 reveal that a probe enhancement effect is present only when an RT effect is also present; indeed, the size of the effect in RTs is close to the effect in probe enhancement. This is addressed in the General discussion, but suggests that capture by a target-irrelevant cue was intermittent, and not occurring on every trial, possibly because observers did not establish a strong target template on a minority of trials
Forest plot for the response time (RT) cuing effects for target-relevant cues and target-irrelevant cues for each target in Experiments 1–6. Cuing effects for individual experiments are arranged from smallest (bottom) to largest (top) based on the size of the target-relevant cue effect. Error bars are the 95% confidence intervals
Forest plot for the enhancement effects in probe identification for target-relevant cues and target-irrelevant cues for each target in Experiments 1–6. Cuing effects for individual experiments are arranged from smallest (bottom) to largest (top) based on the size of the target-relevant cue effect. Error bars are the 95% confidence intervals
General discussion
The present study combined a probe identification task with a spatial cuing task to examine the capture of attention by salient stimuli that were relevant or irrelevant to a target’s feature. This setup allowed probe identification performance the show where attention was focused after the appearance of a cue, so as to examine the influence of top-down settings on the early capture of attention by salient stimuli. Across six experiments, the pattern observed in the RTs and in probe identifications was similar, and showed that the target feature modulated the size of the RT cuing effects and probe enhancement effects. For RTs, cuing effects were present for target-relevant cues and were smaller or absent for target-irrelevant cues. Additionally, probes were identified more when cued by a target-relevant cue than a target-irrelevant cue. Because probe identification was the reason for this study, those results will be the focus below.
Taken as a whole, the results across the six experiments are somewhat mixed and inconclusive, particularly because of a persistent positive probe enhancement effect and cuing effect by onsets in the red target groups, and occasional negative probe enhancement effects for red cues in the onset target groups. The positive cuing effects and probe enhancement effects by the onset cues are suggestive of stimulus-driven capture (e.g., disengagement), because they were elicited by target-irrelevant cues. However, this result does not necessarily support attentional disengagement (Theeuwes et al., 2001), for two reasons: (1) similar positive enhancement effects were not obtained for red cues in the onset target groups; (2) Experiment 2 failed to find a decrease in the probe enhancement as a function of cue-probe SOA.
The negative probe enhancement effect for red cues in the onset target group in Experiment 1, which was present but not statistically significant in several other experiments, suggests observers may have suppressed the location with the target-irrelevant cue. However, Experiments 4 and 5 failed to obtain evidence of suppression at the location cued by the target-irrelevant cues. Thus, the two effects observed across several experiments obscure a single theoretical interpretation. The overall pattern (larger probe enhancement for target-relevant cues than for target-irrelevant cues) is consistent with Folk et al.’s (1992) contingent orienting hypothesis. However, the positive and statistically significant probe enhancement effects by onset cues in the red target groups (except Experiment 6), conflicts with that hypothesis – a point I address below – as such, the theoretical impact of the results must be tempered.
The RT cuing effect and probe enhancement effect by onset cues in the red target groups (and green cues in Experiment 4) was inconsistent with contingent capture, but somewhat consistent with stimulus-driven accounts (e.g., disengagement) of capture. However, the effect was smaller than the effect elicited by red cues (Fig. 7), indicating that top-down settings did exert an influence on the ability of a cue to capture attention. Stimulus-driven accounts also predict positive effects by red cues in the onset target group, which was not observed in any experiment and, if anything, tended to be negative. As such, the onset cuing effects do not appear to be purely stimulus-driven and may have resulted from one of several factors.
First, it is possible that observers in the red target groups adopted a template for ‘red’ as well displaywide settings for ‘onset’ to detect the appearance of the target display (Gibson & Kelsey, 1998; Johnson et al., 2001). Such displaywide settings would establish a relationship between the onset cue and the onset of the target display and may have elicited displaywide contingent capture of attention. Second, because stimuli in the probe task for both target groups included abruptly onset white letters, perhaps these features also entered observers’ attentional settings. For the onset target group, because such features of the letter probes were already used to locate the target, they would not have affected performance; however, for the red target group, these added features of the letter probes would have made onset cues relevant. Lastly, the probe task may have increased working memory load, and in turn may have diminished attentional control, allowing the onset cues to capture attention.
There are issues with each of these possibilities. Regarding the first two involving displaywide settings, if observers adopted a setting for “onset” of the target display or “onset” of white letters, onset cues would be expected to produce nearly as large cuing effects as the target-relevant red cue, which was not the case. Additionally, this cannot explain the cuing effect by green cues in the red target group in Experiment 4. Regarding the third possibility, if working memory load increased, this should have occurred for both target groups. Thus, in the red target group, cuing effects should have been elicited by the red cues, which was not the case.
A fourth and more likely explanation for the positive enhancement effect by onset cues is failure of observers to maintain a target template for “red” across all trials. That is, fluctuations in top-down control may have allowed the onset cue to capture attention on a minority of trials, perhaps when a target template was established only after the target display appeared. This ad hoc explanation follows from Leber’s (2010) finding that pre-trial activity in left middle frontal gyrus (MFG) predicted attentional capture by color singletons during a shape singleton search. The MFG is a region believed to have a role in top-down control over attention in order to prevent distraction (e.g., Corbetta & Shulman, 2002). Leber (2010) found that when pre-trial MFG activity was high, attentional capture by a color singleton was smaller than when pre-trial MFG activity was low. Leber’s (2010) results suggest that observers can establish target templates that modulate the capture of attention, but these templates are not sustained over the course of an experiment and fluctuate from trial-to-trial. Indeed, in Fig. 7, the point plotted for the onset cue/red target combination suggests probe enhancement occurred only when the onset cue elicited an RT cuing effect. Because this effect (in RTs and probe identifications) was smaller than for red cues, this suggests capture by the onset cue was not occurring on all trials, but was intermittent. Thus, observers may have established target templates and ignored irrelevant cues, consistent with contingent orienting, but because a template was not maintained on every trial, capture by onsets occurred occasionally. This conclusion comes from consistently finding cuing effects by irrelevant cues were absent or smaller than for relevant cues, and the meta-analysis showing cued probe identification was better following target relevant cues than target irrelevant cues.
However, two issues were not addressed in this study. First, by using a blocked design, the contingent capture effects may have been elicited through intertrial priming. Specifically, cuing by target-relevant cues may have been contingent on feature similarity between the target on trial n – 1 and the cue on trial n, not a top-down set for target-defining features. Studies have shown intertrial priming inflates cuing effects for target-relevant cues (Belopolsky et al., 2010; Folk & Remington, 2008). However, this effect is difficult to replicate (Eimer & Kiss, 2010; Irons et al. 2012) and, recently, Schoeberl, Goller and Ansorge (2019) failed to find evidence that feature priming could explain contingent capture. Additionally, in a meta-analysis, Büsel, Voracek, and Ansorge (2018; see also Lamy & Kristjánsson, 2013) found no evidence that contingent capture could be explained through intertrial priming. As such, it is unlikely the results from the present experiments were due to intertrial priming.
Secondly, the probe task in the present study used the same whole-report technique as Gaspelin et al. (2015) and Gaspelin and Luck (2018); that is, observers recalled as many letters as possible from the probe display. At issue is that whole report is susceptible to forgetting compared to partial report; hence, subjects may have recognized more letters in the probe display, but some letters may have decayed before subjects could recall them. Thus, it is debatable whether the whole-report probe technique is ideal for studying attentional selection, compared to the partial-report techniques used by Huffman et al. (2018). While this is a limitation of whole report, this cannot explain the increased likelihood of recalling cued probes than uncued probes, which appeared in the same display as the cued probe, in the present experiments. Specifically, even if one or two uncued probes were recognized, but decayed before they could be recalled, the probe enhancement effects clearly show that cued probes were more likely to be recalled from the letter display than uncued probed. Hence, target-relevant cues captured attention, leading to enhancement of the letter probe at that location.
Notwithstanding these two issues and the probe enhancement effect by onset cues in the red target group, this study provides evidence that top-down settings modulate the allocation of attention to salient stimuli. Specifically, cuing effects and probe enhancement effects were larger when elicited by target-relevant cues than target-irrelevant cues. Importantly, the probe results provide evidence that top-down modulation occurs soon after cue appearance, which is a benefit to probe tasks: with a letter probe at each possible target location, one obtains unequivocal evidence where attention is focused within the visual field.
Conclusion
Across six experiments, the results consistently showed greater probe enhancement effects for letters appearing near target-relevant cues than near target-irrelevant cues; hence, the top-down settings for target features modulated the capture of attention by salient items. The results are somewhat consistent with Folk et al.’s (1992) contingent orienting hypothesis; however, there is one important caveat: observers did not maintain a consistent target template, leading to intermittent capture by the onset cues. Although the debate over how top-down, bottom-up, and selection history (Awh, Belopolsky, & Theeuwes, 2012) influences attentional allocation is far from over, the results reported here and the use of probe identification and other attentional measurement tasks should help researchers better understand the spread and locus of attention.
Open Practices Statement
None of the experiments reported in this study was preregistered; however, data or materials used to run the experiments will be made available upon request. Data have not been, nor can be, deposited as this was not spelled out in the consent forms.
Notes
Gaspelin et al.’s (2016) dwelling hypothesis resolves why irrelevant onsets show capture effects in some situations and not others; however, the hypothesis was never applied to color singletons.
ESu = MInvalid - MValid.
\( {SE}_u=\sqrt{\frac{2{s}_p^2\left(1-r\right)}{n}} \)
\( {w}_u=\frac{n}{2{s}_p^2\left(1-r\right)} \)
References
Anderson B. A. (2014). On the precision of goal-directed visual attentional selection. Journal of Experimental Psychology: Human Perception & Performance, 40, 1755-1762. doi: https://doi.org/10.1037/a0037685
Anderson B. A. & Folk C. L. (2010). Variations in the magnitude of attentional capture: Testing a two-process model. Attention, Perception, & Psychophysics, 72, 342–352. doi: https://doi.org/10.3758/APP.72.2.342
Anderson B. A. & Folk C. L. (2012). Dissociating location-specific inhibition and attention shifts: Evidence against the disengagement account of contingent capture. Attention, Perception & Psychophysics, 74, 1183-1198. doi: https://doi.org/10.3758/s13414-012-0325-9
Ansorge, U., & Heumann, M. (2003) Top-down contingencies in peripheral cuing: The roles of color and location. Journal of Experimental Psychology: Human Perception & Performance, 29, 937-948.
Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Science, 16, 437-443. doi: https://doi.org/10.1016/j.tics.2012.06.010
Bacon, W., & Egeth, H. E. (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55, 485-496.
Barras, C., & Kerzel, D. (2016). Nogo stimuli do not receive more attentional suppression or response inhibition than neutral stimuli: Evidence from the N2pc, PD, and N2 components in a spatial cueing paradigm. Frontiers in Psychology, 7:630. doi:https://doi.org/10.3389/fpsyg.2016.00630
Belopolsky, A.V., Kramer, A. F., & Theeuwes, J. (2005). Prioritization by visual transients in visual search. Psychonomic Bulletin & Review, 12, 93-99. doi: https://doi.org/10.3758/BF03196352
Belopolsky, A.V., Schreij, D. & Theeuwes, J. (2010). What is top-down about contingent capture? Attention, Perception & Psychophysics, 72, 326-341. doi: https://doi.org/10.3758/APP.72.2.326
Born, S., Kerzel, D., & Pratt, J. (2015). Contingent capture effects in temporal order judgments. Journal of Experimental Psychology: Human Perception & Performance, 41, 995-1006. doi: https://doi.org/10.1037/xhp0000058
Burnham, B. R., Neely, J. H., Naginsky, Y., & Thomas, M. (2010). Stimulus-driven attentional capture by a static discontinuity between perceptual groups. Jurnal of Experimental Psychology: Human Perception & Performance, 36, 317-329. doi: https://doi.org/10.1037/a0015871
Burnham, B. R. (2007). Displaywide visual features associated with a search display’s appearance can mediate attentional capture. Psychonomic Bulletin & Review, 14, 392-422.
Burra, N., & Kerzel, D. (2013). Attentional capture during visual search is attenuated by target predictability: Evidence from the N2pc, Pd, and topographic segmentation. Psychophysiology, 50, 422-430. doi: https://doi.org/10.1111/psyp.12019
Büsel, C., Voracek, M., & Ansorge, U. (2018). A meta-analysis of contingent-capture effects. Psychological Research. doi. https://doi.org/10.1007/s00426-018-1087-3
Chen, P., & Mordkoff, J.T. (2007). Contingent capture at a very short SOA: Evidence against rapid disengagement. Visual Cognition, 15, 637-646. doi: https://doi.org/10.1080/13506280701317968
Corbetta, M., & Shulman, G. L. (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215.
Eimer, M. (1996). The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology, 99, 225–234.
Eimer, M. Kiss, M. (2010). Top-down search strategies determine attentional capture in visual search: Behavioral and electrophysiological evidence. Attention, perception & Psychophysics, 72, 951-962.
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175-191.
Folk, C. L., Leber, A. B., & Egeth, H. E. (2002). Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics, 64, 741-753.
Folk, C. L., Leber, A. B., & Egeth, H. E. (2006).Top-down control settings and the attentional blink: Evidence for nonspatial contingent capture. Visual Cognition, 16, 616-642. Doi: https://doi.org/10.1080/13506280601134018
Folk, C. L., & Remington, R. W. (1998). Selectivity by irrelevant featural singletons: Evidence for two forms of attentional capture. Journal of Experimental Psychology: Human Perception & Performance, 24, 847-858. doi: https://doi.org/10.1080/13506280500193545
Folk, C. L., & Remington, R. W. (2006). Top-down modulation of preattentive processing: Testing the recovery account of contingent capture. Visual Cognition, 14, 445-465.
Folk, C. L., & Remington, R. W. (2008). Bottom-up priming of top-down attentional control settings. Visual Cognition, 16, 215-231.
Folk, C. L. & Remington, R. (2010). A critical evaluation of the disengagement hypothesis. Acta Psychologica, 135, 103-105.
Folk, C. L., Remington, R. W., & Johnston, J. C. (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception & Performance, 18, 1030-1044.
Folk, C. L., Remington, R. W., & Wright, J. H. (1994). The structure of attentional control: Contingent attentional capture by apparent motion, abrupt onset, and color. Journal of Experimental Psychology: Human Perception and Performance, 20, 317-329.
Franconeri, S. L., Simons, D. J., & Junge, J. A. (2005). Searching for stimulus-driven shifts of attention. Psychonomic Bulletin & Review, 11, 876-881.
Gaspar, J. M., & McDonald, J. J. (2014). Suppression of salient objects prevents distraction in visual search. The Hournal of Neuroscience, 34, 5658-5666. doi: https://doi.org/10.1523/JNEUROSCI.4161-13.2014
Gaspelin, N., Leonard, C. J., & Luck, S. J. (2015). Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychological Science, 26, 1740-1750. doi: https://doi.org/10.1177/0956797615597913
Gaspelin, N., & Luck, S. J. (2018). Distinguishing among potential mechanisms of singleton suppression. Journal of Experimental Psychology: Human Perception & Performance, 44, 626-644. doi: https://doi.org/10.1037/xhp0000484
Gaspelin, N., Ruthruff, E., & Lien, M.-C. (2016). The problem of latent attentional capture: Easy visual search conceals capture by task-irrelevant abrupt onsets. Journal of Experimental Psychology: Human Perception & Performance, 42, 1104-1120. https://doi.org/10.1037/xhp0000214
Gibson, B. S., & Kelsey, (1998). Stimulus-driven attentional capture is contingent on attentional set for displaywide visual features. Journal of Experimental Psychology: Human Perception & Performance, 24, 699-706.
Hickey, C., Di Lollo, V., & McDonald, J. J. (2009). Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience, 21, 760-775.
Hollands, J. G., & Jarmasz, J. (2010). Revisiting confidence intervals for repeated measures designs. Psychonomic Bulletin & Review, 17, 135-138. doi: https://doi.org/10.3758/PBR.17.1.135
Huffman, G., Antinucci, V. M., & Pratt, J. (2018). The illusion of control: Sequential dependencies underlie contingent attentional capture. Psychonomic Bulletin & Review 25, 2238–2244. doi:https://doi.org/10.3758/s13423-017-1422-5
Irons, J. L., Folk, C. L., & Remington, R. W. (2012). All set! Evidence of simultaneous attentional control settings for multiple target colors. Journal of Experimental Psychology: Human Perception & Performance, 38, 758-775. doi:https://doi.org/10.1037/a0026578
Johnson, J. D., Hutchison, K. A., & Neill, W. T. (2001). Attentional capture by irrelevant color singletons. Journal of Experimental Psychology: Human Perception & Performance, 27, 841-847.
Jonides, J. (1981). Voluntary vs. automatic control over the mind’s eye’s movements. In J. B. Long & A. D. Baddeley (Eds.), Attention and performance IX (pp. 187-203). Hillsdale, NJ: Erlbaum.
Kim, M.-S., & Cave, K. R. (1999). Top-down and bottom-up attentional control: On the nature of the interference from a salient distractor. Perception & Psychophysics, 61, 1009-1023.
Lamy, D., & Egeth, H. E. (2003). Attentional capture in singleton-detection and feature-search modes. Journal of Experimental Psychology: Human Perception & Performance, 29, 1021-1035.
Lamy, D. & Kristjánsson, Á. (2013). Is goal-directed attentional guidance just intertrial priming? A review. Journal of Vision, 13, 14.
Lamy, D., Leber, A., & Egeth, H. E. (2004). Effects of task relevance and stimulus-driven salience in feature-search mode. Journal of Experimental Psychology: Human Perception and Performance, 30, 1019–1031. doi:https://doi.org/10.1037/0096-1523.30.6.1019
Lamy, D., Leber, A. B., & Egeth, H. E. (2012). Selective attention: Experimental psychology. In I. B. Weiner (Ed.), Handbook of psychology (2nd ed., Vol. 4, pp. 265–294). New York, NY: Wiley. doi:https://doi.org/10.1002/9781118133880.hop204010
Leber, A. B. (2010). Neural predictors of within-subject fluctuations in attentional control. Journal of Neuroscience, 30 , 11458-11465. doi: https://doi.org/10.1523/JNEUROSCI.0809-10.2010
Leber, A. B., & Egeth, H. E. (2006). It’s under control: Top-down search strategies can override attentional capture. Psychonomic Bulletin & Review, 13, 132-138.
Lien, M.-C., Ruthruff, E., Goodin, Z., & Remington, R. W. (2008). Contingent attentional capture by top-down control settings: Converging evidence from event-related potentials. Journal of Experimental Psychology: Human Perception & Performance, 34, 509-530.
Lien, M.-C., Ruthruff, E., & Johnston, J. C. (2010). Attention capture with rapidly changing attentional control settings. Journal of Experimental Psychology: Human Perception & Performance, 36, 1-16.
Lipsey, M. W., & Wilson, D. B. (2001). Practical meta-analysis. Sage Publications: Thousand Oaks, CA.
Luck, S. J. (2006). An introduction to the event-related potential technique. Cambridge: The MIT Press.
Luck, S. J., & Hillyard, S. A. (1994a). Electrophysiological correlates of feature analysis during visual search. Psychophysiology, 31, 291–308.
Luck, S. J., & Hillyard, S. A. (1994b). Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception & Performance, 20, 1000–1014.
Posner, M. I. (1980). Orienting of attention, the VIIth Sir Frederic Barlett lecture. Quarterly Journal of Experimental Psychology, 32, 3–25.
Pratt, J., & McAuliffe, J. (2002). Determining whether attentional control settings are inclusive or exclusive. Perception & Psychophysics, 64, 1361-1370.
Pratt, J., Sekuler, A. B., & McAuliffe, J. (2001). The role of attentional set on attentional cueing and inhibition of return. Visual Cognition, 8, 33-46. doi: https://doi.org/10.1080/13506280042000018
Sawaki, R., & Luck, S. J. (2010). Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception & Psychophysics, 72, 1455-1470. doi: https://doi.org/10.3758/APP.72.6.1455
Schreij, D., Owens, C., & Theeuwes, J. (2008). Abrupt onsets capture attention independent of top-down control settings. Perception & Psychophysics, 70, 208–218.https://doi.org/10.3758/PP.70.2.208
Schreij, D., Theeuwes, J., & Olivers, C. N. L. (2010a) Irrelevant onsets cause inhibition of return regardless of attentional set. Attention, Perception & Psychophysics, 72, 1725-1729. doi: https://doi.org/10.3758/APP.72.7.1725
Schreij, D., Theeuwes, J., & Olivers, C. N. L. (2010b). Abrupt onsets capture attention independent of top-down control settings II: Additivity is no evidence for filtering. Attention, Perception, & Psychophysics, 72, 672–682. doi:https://doi.org/10.3758/APP.72.3.672
Schoeberl, T., Goller, F., & Ansorge, U. (2019). Top-down matching singleton cues have no edge over top-down matching nonsingletons in spatial cueing. Psychonomic Bulletin & Review, 26, 241-249. doi: https://doi.org/10.3758/s13423-018-1499-5
Theeuwes, J. (1992). Perceptual selectivity of color and form. Perception & Psychophysics, 51, 599-606.
Theeuwes, J. (1994). Stimulus-driven attentional capture and attentional set: Selective search for color and abrupt onsets. Journal of Experimental Psychology: Human Perception & Performance, 20, 799-806.
Theeuwes, J. (2004). Top-down search strategies cannot override attentional capture. Psychonomic Bulletin & Review, 11, 65-70. doi: https://doi.org/10.3758/BF03206462
Theeuwes, J. (2010). Top-down and bottom-up control of visual selection. Acta Psychologica, 123, 77-99.
Theeuwes, J., Atchley, P., & Kramer, A.F. (2000). On the time course of top-down and bottom-up control of visual attention (p. 105-125). In S. Monsell & J. Driver (Eds.). Attention & performance (Vol 18). Cambridge: MIT Press.
Theeuwes, J., & Godijn, R. (2002). Irrelevant singletons capture attention: Evidence from inhibition of return. Perception & Psychophysics, 64, 764-770.
Theeuwes, J., Kramer, A. F., Hahn, S., & Irwin, D. E. (1998). Our eyes do not always go where we want them to go: Capture of the eyes by new objects. Psychological Science, 9, 379-385.
Turatto, M., & Galfano, G. (2001). Attentional capture by color without any relevant attentional set. Perception & Psychophysics, 63, 286-297.
Turatto, M., Galfano, G., Gardini, S., & Mascetti, G. G. (2004). Stimulus-driven attentional capture: An empirical comparison of display-size and distance methods. Quarterly Journal of Experimental Psychology: Human Experimental Psychology, 57A, 297-324.
Wright, R. D., & Ward, L. M. (2008). Orienting of attention. New York: Oxford University Press.
Yantis, S. (2000). Goal-directed and stimulus-driven determinants of attentional capture (tutorial). In S. Monsell and J. Driver (Eds). Control of cognitive processes: Attention and performance XVIII. (pp. 73 – 103). Cambridge, MA: MIT Press.
Yantis, S., & Egeth, H. E. (1999). On the distinction between visual salience and stimulus-driven attentional capture. Journal of Experimental Psychology: Human Perception & Performance, 25, 661-676.
Yantis, S., & Jonides, J. (1984). Abrupt visual onsets and selective attention: Evidence from visual search. Journal of Experimental Psychology: Human Perception & Performance, 10, 601-621.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Public significance
Human attention may be directed toward objects of importance (top-down control) and may be involuntarily drawn toward salient objects (bottom-up processing). Using a new approach to measure the focus of attention, the present study showed that salient but non-important items can be ignored early in visual processing. This study may be important for development of visual displays, computer interfaces, and even video games.
Rights and permissions
About this article
Cite this article
Burnham, B.R. Evidence for early top-down modulation of attention to salient visual cues through probe detection. Atten Percept Psychophys 82, 1003–1023 (2020). https://doi.org/10.3758/s13414-019-01850-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-019-01850-0