Abstract
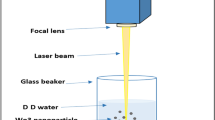
Colloidal solution precipitates obtained during laser ablation of tungsten in water and containing nanostructured metal oxides are studied using X-ray diffraction and scanning electron microscopy. The nanostructure composition and morphology are analyzed. It is shown that the material composing nanostructures is X-ray amorphous, i.e., the particle size does not exceed 1–2 nm. The high degree of the structure surface development implies prospects of their use as substrata when analyzing the composition of various materials by surface-enhanced Raman scattering.
Similar content being viewed by others
References
R. Aroca, Surface Enhanced Vibrational Spectroscopy (Wiley, Chichester, 2006).
Y. S. Yamamoto and T. Itoh, J. Raman Spectrosc. 47, 78 (2016).
J. R. Lombardi and R. L. Birke, J. Phys.Chem. C 118, 11120 (2014).
W. J. Zhao and Y. Ozaki, J. Raman Spectrosc. 47, 51 (2016).
Y. Wang, W. Ruan, J. Zhang, et al., J. Raman Spectrosc. 40, 1072 (2016).
X. Fu, F. Bei, X. Wang, et al., Mater. Lett. 63, 185 (2009).
B. Mondal and S. K. Saha, Chem. Phys. Lett. 497, 89 (2010).
W. Q. Li, G. Wang, et al., Nanoscale 7, 15487 (2015).
Yu-Luen Deng and Yi-Je Juang, Biosens. Bioelectron. 53, 37 (2014).
V. T. Karpukhin, I. I. Klimovskii, V. M. Batenin, et al., Lasers on Self-Terminating Transitions in Metal Atoms, Vol. 2 (Fizmatlit, Moscow, 2011) [in Russian].
V. T. Karpukhin, M. M. Malikov, T. I. Borodina, et al., in Chemical and StructureModification of Polymers, Ed. by K. Pyrzynski, G. Nyszko, and G. E, Zaikov (Apple Academic Press, Oakville, 2015), p.187.
A. Yu. Varaksin, M. E. Romash, and V. N. Kopeitsev, High Temp. 47, 836 (2009).
A. Yu. Varaksin, M. E. Romash, V. N. Kopeitsev, and M. A. Gorbachev, High Temp. 48, 918 (2010).
P. Minutolo, G. Rusciano, L. A. Sro, et al., Proc. Combust. Inst. 33, 649 (2011).
D. A. Mamichev, I. E, Kuznetsov, N. E. Maslova, and M. L. Zanaveskin, Molecular medicine, No. 6, 3 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.T. Karpukhin, M.M. Malikov, T.I. Borodina, G.E. Val’yano, M.A. Kazaryan, 2018, published in Kratkie Soobshcheniya po Fizike, 2018, Vol. 45, No. 7, pp. 46–50.
About this article
Cite this article
Karpukhin, V.T., Malikov, M.M., Borodina, T.I. et al. Morphology of Tungsten Nanooxides, Synthesized by Laser Ablation of Metal in Water. Bull. Lebedev Phys. Inst. 45, 223–225 (2018). https://doi.org/10.3103/S1068335618070072
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068335618070072