Abstract
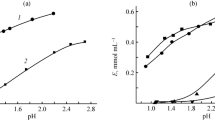
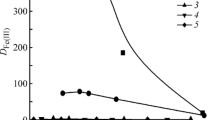
The possibility of the additional purification of ammonia rhenium desorbates with respect to molybdenum in the course of the sorption recovery of rhenium from Mo-containing solutions with the help of Purolite A170 and Purolite A172 weak base anion-exchange resins is considered. The pH-dependence of sorption of Re(VII) and Mo(VI) on these anion-exchange resins is investigated in static conditions with the 1 M (NH4)2SO4 background in the solution. It is shown that the range of pH, in which anion-exchange resins retain the ability to sorb Re(VII), is also spread to a weakly basic region. A substantial decrease in the adsorption of Re(VII) starts already with an increase in pH above 7.5. The capacity of anion-exchange resins with respect to Mo(VI) starts to decrease noticeably with an increase in pH of solutions above 5.0, and molybdenum almost ceases to sorb by both anion-exchange resins upon reaching pH ~ 7.0. In order to decrease the Mo(VI) content in rhenium desorbates with the sorption recovery of Re(VII) from Mo-containing solutions on weak base anion-exchange resins, the following flowsheet is suggested. Initially, the main amount of sorbed Mo(VI) is desorbed by contacting the saturated anion-exchange resin with the ammonium sulfate solution upon mixture stirring and holding constant pH of the solution in limits of 7.0–7.5 due to the addition of dosed amounts of ammonia solution. Then anion-exchange resin is separated from the ammonium sulfate solution containing Mo(VI), washed with water, and Re(VII) is desorbed by ammonium solution in dynamic conditions. The verification of the proposed method for the resins saturated by sorption from the model solution of the composition, g/L, 98 H2SO4, 4 Mo(VI), and 0.5 Re(VII) showed the occurrence of desorption of no less than 90% sorbed molybdenum during the treatment of anion-exchange resins with ammonium sulfate solution. Herewith, concentration ratio Re(VII) : Mo(VI) in ammoniacal rhenium desorbates when using A170 anion-exchange resin increases 11-fold and when using A172 anion-exchange resin, it increases 20-fold compared with that attained without the additional washing of Mo(VI). Losses of Re(VII) with the Mo-containing desorbate (reversible) do not exceed 5.2% of the amount of sorbed Re(VII).
Similar content being viewed by others
References
Palant, A.A., Troshkina, I.D., and Chekmarev, A.M., Metallurgiya reniya (Metallurgy of Rhenium), Moscow: Nauka, 2007.
Blazy, P., Jdid, E.A., Floreancig, A., and Mottet, B., Selective recovery of rhenium from gas-scrubbing solutions of molybdenite roasting using direct precipitation and separation on resins, Separat. Sci. Technol, 1993, vol. 28, nos. 11–12, pp. 2073–2096.
Juneja, J.M., Singh, S., and Bose, D.K., Investigations on the extraction of molybdenum and rhenium values from low grade molybdenite concentrate, Hydrometallurgy, 1996, vol. 41, nos. 2–3, p. 201.
Shariat, M.H. and Hassani, M., Rhenium recovery from Sarcheshmeh molybdenite concentrate, J. Mater. Process. Technol., 1998, vol. 74, nos 1-3, p. 243.
Tarasov, A.V., Besser, A.D., and Gedgagov, E.I., Integrated technology for processing rhenium-containing molybdenite concentrates to recover molybdenum and rhenium into commercial products, Miner. Process. Extract. Metall. Rev., 2001, vol. 22, no. 2, p. 509.
Anderson, C.D., Taylor, P.R., and Anderson, C.G., Extractive metallurgy of rhenium: a review, Miner. Metall. Process, 2013, vol. 30, no. 1, pp. 59–73.
Kim, H.S., Parka, J.S., Seo, S.Y., Trana, T., and Kima, M.J., Recovery of rhenium from a molybdenite roaster fume as high purity ammonium perrhenate, Hydrometallurgy, 2015, vol. 156, pp. 158–164.
Lebedev, K.B., Rozmanov, V.M., and Ponamarev, V.P., Revisiting the choice of sorbent for recovery of rhenium from aqueous solutions, Zh. Prikl. Khim., 1971, vol. 44, no. 3, p. 296.
Rumyantsev, V.K., Vol’dman, S.G., and Kulakova, V.V., Accompanying recovery of rhenium in processing of molybdenum concentrates, Tsvetn. Met., 1991, no. 3, p. 33.
Kholmogorov, A.G., Kononova, O.N., Kachin, S.V., Ilyichev, S.N., Kiyuchkov, V.V., Kalyakina, O.P., and Pashkov, G.L., Ion exchange recovery and concentration of rhenium from salt solutions, Hydrometallurgy, 1999, vol. 51, no. 1, p. 19.
Peganov, V.A. and Molchanova, T.V., Sorption processes in hydrometallurgy of refractory metals, Atom. Energ., 2001, vol. 90, no. 3, pp. 192–199.
Paretskii, V.M., Besser, A.D., and Gedgagov, E.I., Ways to increase the production of rhenium from ore and anthropogenic raw materials, Tsvetn. Met., 2008, no. 10, pp. 17–21.
Jermakowicz-Bartkowiak, D. and Kolarz, B.N., Poly(4-vinylpyridine) resins towards perrhenate sorption and desorption, React. Funct. Polymer., 2011, vol. 71, no. 2, pp. 95–103.
Laatikainen, M., Virolainen, S., Paatero, E., and Sainio, T., Recovery of ReO4 - by weakly basic anion exchangers, Separat. Purificat. Technol., 2015, vol. 153, pp. 19–28.
Blokhin, A.A., Mal’tseva, E.E., Panchishina, L.B., and Murashkin, Yu.V., Ion exchange recovery of rhenium from molybdenum-containing sulfuric acid solutions, Tsvetn. Met., 2009, no. 7, pp. 53–56.
Abisheva, Z.S., Zagorodnyaya, A.N., Bekturganov, N.S., Ospanov, E.A., and Ospanov, N.A., Study of sorption of rhenium from industrial solutions of washing sulfuric acid of the Balkhash copper smeltery on the A170 anion-exchange resin, Tsvetn. Met., 2012, no. 7, pp. 57–61.
Nebeker, N. and Hiskey, J.B., Recovery of rhenium from copper leach solution by ion exchange, Hydrometallurgy, 2012, vol. 125–126, pp. 64–68.
Mal’tseva, E.E., Blokhin, A.A., Pleshkov, M.A., Murashkin, Yu.V., and Mikhaylenko, M.A., Sorption recovery of rhenium in hydrometallurgical processing of molybdenite concentrates using Purolite A170 and Purolite A172 weakly basic anion-exchange resins, Tsvetn. Met., 2014, no. 6, pp. 52–58.
Virolainen, S., Laatikainen, M., and Sainio, T., Ion exchange recovery of rhenium from industrially relevant sulfate solutions: Single column separations and modeling, Hydrometallurgy, 2015, vol. 158, pp. 74–82.
Helferich, F., Ionenaustauscher. Band I. Gundlagen Struktur—Herstellung-Theorie, Weinheim: Chemie, 1959.
Busev, A.I., Tiptsova, V.G., and Ivanov, V.M., Manual of Analytical Chemistry of Rare Elements, Moscow: Khimiya, 1978.
Aveston, J., Anacker, E.W., and Johnson, J.S., Hydrolysis of molybdenum (VI). Ultracentrifugation, acidity measurements, and Raman spectra of polymolybdates, Inorg. Chem., 1964, vol. 3, no. 5, pp. 735–746.
Olazabal, M.A., Orive, M.M., Fernández, L.A., and Madariaga, J.M., Selective extraction of vanadium(V) from solutions containing molybdenum(VI) by ammonium salts dissolved in toluene, Solvent Extract. Ion Exchang., 1992, vol. 10, no. 4, pp. 623–635.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.E. Maltseva, A.A. Blokhin, Yu.V. Murashkin, M.A. Mikhaylenko, 2017, published in Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgiya, 2017, No. 4, pp. 30–38.
About this article
Cite this article
Maltseva, E.E., Blokhin, A.A., Murashkin, Y.V. et al. An increase in purity of ammonium perrhenate solutions with respect to molybdenum(IV) with the sorption recovery of rhenium(VII) from Mo-containing solutions. Russ. J. Non-ferrous Metals 58, 463–469 (2017). https://doi.org/10.3103/S1067821217050091
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821217050091