Abstract
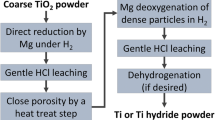
Fine Ti powders were prepared through magnesiothermic reduction in TiO2–Mg–Ca mixtures under 4 MPa of Ar followed by acid leaching (with HCl or HNO3) and characterized by XRD, SEM/EDS, and chemical analysis. Thus prepared Ti powders exhibited the specific surface ranging between 7.0 and 30.0 m2/g. The produced Ti powders can find their application in pyrotechnics, powder metallurgy, and as raw material for SHS of inorganic compounds.
Similar content being viewed by others
References
Titanium and Titanium Alloys: Fundamentals and Applications, Leyens, C. and Peters, M., Eds., Weinheim: Wiley–VCH, 2003. doi.org/10.1179/095066090790323984
Froes, F.H. and Eylon, D., Powder metallurgy of titanium alloys, Int. Mater. Rev., 1990, vol. 35, no. 3, pp. 162–182. doi 10.1179/095066090790323984
Kroll, W.J., The production of ductile titanium, Trans. Electrochem. Soc., 1940, vol. 78, no. 1, pp. 35–47. doi 10.1149/1.3071290
Du, J., Research progress of titanium production technology, Rare Met. Mater. Eng., 2008, vol. 37, no. 10, pp. 1872–1875. doi 10.1007/s11771-012-1293-x
Ogasawara, T., Progress of the titanium production technology in Japan and future prospects of the field, Jpn. Titanium Soc., 2005, vol. 53, no. 2, pp. 103–108.
Zheng, H., Ito, H., and Okabe, T.H., Production of titanium powder by the calciothermic reduction of titanium concentrates or ore using the preform reduction process, Mater. Trans., 2007, vol. 48, no. 8, pp. 2244–2251. doi 10.2320/matertrans.MER2007115
Okabe, T.H., Odab, T., and Mitsuda, Y. Titanium powder production by preform reduction process (PRP), J. Alloys Comp., 2004, vol. 364, nos. 1–2, pp. 156–163. doi.org/10.1016/S0925-8388(03)00610-8
Chen, G.Z., Fray, D.J., and Farthing, T.W., Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride, Nature, 2000, vol. 407, no. 5, pp. 361–364. doi 10.1038/35030069
Suzuki, R.O., Teranuma, K., and Ono, K., Calciothermic reduction of titanium oxide by electrolysis in molten CaCl2, Metall. Mater. Trans. B, 2003, vol. 34, no. 6, pp. 287–295. doi 10.1007/s11663-003-0074-1
Nersisyan, H.H., Won, H.I., Won, C.W., Jo, A.J., and Kim, H., Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis, Chem. Eng. J., 2014, vol. 235, no. 1, pp. 67–74. doi.org/10.1016/j.cej.2013.08.104
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Vershinnikov, V.I., Ignat’eva, T.I., Aleshin, V.V. et al. Fine Ti Powders Through Metallothermic Reduction in TiO2–Mg–Ca Mixtures. Int. J Self-Propag. High-Temp. Synth. 27, 55–59 (2018). https://doi.org/10.3103/S1061386218010120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386218010120