Abstract
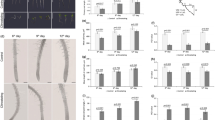
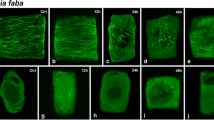
In order to study the role of posttranslational α-tubulin acetylation in the realization of stressinduced autophagy, levels of α-tubulin acetylation in Arabidopsis thaliana plants subjected to abiotic stress, such as salt and osmotic stresses, starvation, or ultraviolet B irradiation, were determined by Western blot analysis. It was shown a significant increase in the levels of acetylated α-tubulin under autophagy development, moreover, a synergistic action of stressful factors and E-64, an autophagy inhibitor, attenuated this increase. In order to study tissue-specific character of this modification, an immunohistochemical analysis of α-tubulin acetylation in A. thaliana seedlings was conducted. These experiments demonstrated the tissuespecific character of α-tubulin acetylation induced by stress, particularly, it is most markedly manifested in young and meristematic tissues, as well as in root tissues (root cap, epidermis and pericycle cells). Obtained results can serve as a proof of the regulatory role of α-tubulin acetylation in the realization of autophagy as an adaptive response to the influence of stressful stimuli.
Similar content being viewed by others
References
Avin-Wittenberg, T., Honig, A., and Galili, G., Variations on a theme: plant autophagy in comparison to yeast and mammals, Protoplasma, 2012, vol. 249, no. 2, pp. 285–299. doi 10.1007/s00709-011-0296-z
Liu, Y. and Bassham, D.C., Autophagy: pathways for self-eating in plant cells, Annu. Rev. Plant Biol., 2012, vol. 63, pp. 215–237. doi. org/10.1146/annurevarplant-042811-105441
Michaeli, S., Galili, G., Genschik, P., Fernie, A.R., and Avin-Wittenberg, T., Autophagy in plants-what’s new on the menu?, Trends Plant Sci., 2016, vol. 21, no. 2, pp. 134–144. doi 10.1016/j.tplants.2015.10.008
Bassham, D.C., Laporte, M., Marty, F., Moriyasu, Y., Ohsumi, Y., Olsen, L.J., and Yoshimoto, K., Autophagy in development and stress responses of plants, Autophagy, 2006, vol. 2, no. 1, pp. 2–11.
Batoko, H., Dagdas, Y., Baluska, F., and Sirko, A., Understanding and exploiting autophagy signaling in plants, Essays Biochem., 2017, vol. 61, no. 6, pp. 675–685. doi 10.1042/EBC20170034
Wang, P., Mugume, Y., and Bassham, D.C., New advances in autophagy in plants: regulation, selectivity and function, Semin. Cell Dev. Biol., 2017, pii S1084-9521(17)30129-5. doi 10.1016/j.semcdb.2017.07.018
Mackeh, R., Perdiz, D., Lorin, S., Codogno, P., and Pöus, C., Autophagy and microtubules-new story, old players, J. Cell Sci., 2013, vol. 126, no. 5, pp. 1071–1080. doi 10.1242/jcs.115626
Köchl, R., Hu, X.W., Chan, E.Y., and Tooze, S.A., Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes, Traffic, 2006, vol. 7, no. 2, pp. 129–145. doi 10.1111/j.1600-0854.2005.00368.x
Monastyrska, I., Rieter, E., Klionsky, D.J., and Reggiori, F., Multiple roles of the cytoskeleton in autophagy, Biol. Rev. Camb. Philos. Soc., 2009, vol. 84, no. 3, pp. 431–448. doi 10.1111/j.1469-185X.2009.00082.x
Geeraert, C., Ratier, A., Pfisterer, S.G., Perdiz, D., Cantaloube, I., Rouault, A., Pattingre, S., Proikas-Cezanne, T., Codogno, P., and Pöus, C., Starvationinduced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation, J. Biol. Chem., 2010, vol. 285, no. 31, pp. 24184–24194. doi 10.1074/jbc.M109.091553
Xie, R., Nguyen, S., McKeehan, W.L., and Liu, L., Acetylated microtubules are required for fusion of autophagosomes with lysosomes, BMC Cell Biol., 2010, vol. 11. doi.org/10.1186/1471-2121-11-89
Lytvyn, D., Yemets, A., Bergounioux, C., and Blume, Ya.B., Development of autophagy in BY-2 (Nicotiana tabacum) cells is accompanied by acetylation of α-tubulin, Rep. Natl. Acad. Sci. Ukraine, 2013, vol. 5, pp. 179–185.
Olenieva, V., Lytvyn, D., Yemets, A., Bergounioux, C., and Blume, Y., Tubulin acetylation accompanies autophagy development induced by different abiotic stimuli in Arabidopsis thaliana, Cell Biol. Int., 2017. doi 10.1002/cbin.10842
Lytvyn, D.I. and Blume, Ya.B., Microtubular Cytoskeleton in Autophagy and Programmed Cell Death Development in Plants, New York: Nova Sci. Publ., 2016, pp. 1–34.
Tran, H.T., Nimick, M., Uhrig, R.G., Templeton, G., Morrice, N., Gourlay, R., DeLong, A., and Moorhead, G.B., Arabidopsis thaliana histone deacetylase 14 (HDA14) is an α-tubulin deacetylase that associates with PP2A and enriches in the microtubule fraction with the putative histone acetyltransferase ELP3, Plant J., 2012, vol. 71, no, pp. 263–272. doi 10.1111/j.1365-313X.2012.04984.x
Ding, Y. and Mou, Z., Elongator and its epigenetic role in plant development and responses to abiotic and biotic stresses, Front. Plant Sci., 2015, vol. 6. doi 10.3389/fpls.2015.00296
Olenieva, V., Lytvyn, D., Yemets, A., and Blume, Ya.B., Influence of UV-B on expression profiles of genes involved in the development of autophagy by means of microtubules, Rep. Natl. Acad. Sci. Ukraine, 2018, vol. 1, pp. 100–109. doi 10.15407/dopovidi2018.01.100
Lytvyn, D.I., Yemets, A.I., and Blume, Y.B., UV-B overexposure induces programmed cell death in a BY-2 tobacco cell line, Environ. Exp. Bot., 2010, vol. 68, no. 1, pp. 51–57. doi 10.1016/j.envexpbot.2009.11.004
Giannoutsou, E., Galatis, B., Zachariadis, M., and Apostolakos, P., Formation of an endoplasmic reticulum ring associated with acetylated microtubules in the angiosperm preprophase band., Cytoskeleton, 2012, vol. 69, no. 4, pp. 252–265. doi 10.1002/cm.21020
Smertenko, A., Blume, Y., Viklicky, V., Opatrny, Z., and Draber, P., Post-translational modifications and multiple tubulin isoforms, Planta, 1997, vol. 201, no. 3, pp. 349–358. doi 10.1007/s004250050077
Nakagawa, U., Suzuki, D., Ishikawa, M., Sato, H., Kamemura, K., and Imamura, A., Acetylation of α-tubulin on Lys40 is a widespread post-translational modification in angiosperms, Biosci. Biotechnol. Biochem., 2013, vol. 77, no. 7, pp. 1602–1605. doi 10.1271/bbb.130261
Nakagawa, U., Kamemura, K., and Imamura, A., Regulated changes in the acetylation of α-tubulin on Lys40 during growth and organ development in fast plants, Brassica rapa L., Biosci. Biotechnol. Biochem., 2013, vol. 77, no. 11, pp. 2228–2233. doi.org/10.1271/bbb.130475
McEwan, D.G. and Dikic, I., The three musketeers of autophagy: phosphorylation, ubiquitylation and acetylation, Trends Cell Biol., 2011, vol. 21, no. 4, pp. 195–201. doi 10.1016/j.tcb.2010.12.006
Popelka, H. and Klionsky, D.J., Post-translationally modified structures in the autophagy machinery: an integrative perspective, FEBS J., 2015, vol. 282, no. 18, pp. 3474–88. doi 10.1111/febs.13356
Dubrovsky, J.G., Doerner, P.W., Colyn-Carmona, A., and Rost, T.L., Pericycle cell proliferation and lateral root initiation in Arabidopsis, Plant Physiol., 2000, vol. 124, no. 4, pp. 1648–1657. doi org/10.1104/pp.124.4.1648
Xiong, X., Xu, D., Yang, Z., Huang, H., and Cui, X., A single amino-acid substitution at lysine 40 of an Arabidopsis thaliana-tubulin causes extensive cell proliferation and expansion defects, J. Integr. Plant Biol., 2013, vol. 55, no. 3, pp. 209–220. doi 10.1111/jipb.12003
Dalwadi, U. and Yip, C.K., Structural insights into the function of Elongator, Cell Mol. Life Sci., 2018. doi 10.1007/s00018-018-2747-6
DeFraia, C. and Mou, Z., The role of the Elongator complex in plants, Plant Signal. Behav., 2011, vol. 6, no. 1, pp. 19–22.
Creppe, C., Malinouskaya, L., Volvert, M.L., Gillard, M., Close, P., Malaise, O., Laguesse, S., Cornez, I., Rahmouni, S., Ormenese, S., Belachew, S., Malgrange, B., Chapelle, J.P., Siebenlist, U., Moonen, G., Chariot, A., and Nguyen, L., Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin, Cell, 2009, vol. 136, no. 3, pp. 551–364. doi 10.1016/j.cell.2008.11.043
Nelissen, H., Fleury, D., Bruno, L., Robles, P., DeVeylder, L., Traas, J., Micol, J.L., Van Montagu, M., Inze, D., and Van Lijsebettens, M., The elongate mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth, Proc. Natl. Acad. Sci. U. S. A., 2005, vol. 102, no. 21, pp. 7754–7759. doi 10.1073/pnas.0502600102
Lytvyn, D.I., Fedyna, V.D., Yemets, A.I., and Blume, Y.B., α-Tubulin acetylation influences on microtubule protein microenvironment under autophagy development in tobacco cells, in Factors in Exp. Evolution of Organisms, 2015, vol. 17, pp. 65–69.
Olenieva, V., Lytvyn, D., Yemets, A., and Blume, Ya.B., Influence of sucrose starvation, osmotic and salt stresses on expression profiles of genes involved in the development of autophagy by means of microtubules, Bull. Vavilov Soc. Genet. Breed. Ukraine, 2018, vol. 16, no. 2, pp. 174–180.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.I. Lytvyn, V.D. Olenieva, A.I. Yemets, Ya.B. Blume, 2018, published in Tsitologiya i Genetika, 2018, Vol. 52, No. 4, pp. 3–13.
About this article
Cite this article
Lytvyn, D.I., Olenieva, V.D., Yemets, A.I. et al. Histochemical Analysis of Tissue-Specific α-Tubulin Acetylation as a Response to Autophagy Induction by Different Stress Factors in Arabidopsis thaliana. Cytol. Genet. 52, 245–252 (2018). https://doi.org/10.3103/S0095452718040059
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452718040059