Abstract
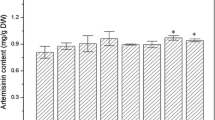
Artemisinin, a sesquiterpene lactone endoperoxide derived from Artemisia annua L., is the most effective antimalarial drug. In an effort to increase the artemisinin production, abscisic acid (ABA) with different concentrations (1, 10 and 100 µM) was tested by treating A. annua plants. As a result, the artemisinin content in ABA-treated plants was significantly increased. Especially, artemisinin content in plants treated by 10 µM ABA was 65% higher than that in the control plants, up to an average of 1.84% dry weight. Gene expression analysis showed that in both the ABA-treated plants and cell suspension cultures, HMGR, FPS, CYP71AV1 and CPR, the important genes in the artemisinin biosynthetic pathway, were significantly induced. While only a slight increase of ADS expression was observed in ABA-treated plants, no expression of ADS was detected in cell suspension cultures. This study suggests that there is probably a crosstalk between the ABA signaling pathway and artemisinin biosynthetic pathway and that CYP71AV1, which was induced most significantly, may play a key regulatory role in the artemisinin biosynthetic pathway.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- ADS :
-
amorpha-4,11-diene synthase gene
- CPR :
-
cytochrome P450 reductase gene
- CYP71AV1 :
-
amorpha-4,11-diene C-12 oxidase gene
- FPS :
-
farnesyl diphosphate synthase gene
- HMGR :
-
3-hydroxy-3-methylglutaryl coenzyme A reductase gene
- HPLC-ELSD:
-
high performance liquid chromatography coupled with evaporative light scattering detection
References
Abdin M.Z., Israr M., Rehman R.U. & Jain S.K. 2003. Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med. 69: 289–299.
Baldi A. & Dixit V.K. 2008. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour. Technol. 99: 4609–4614.
Barrero J.M., Rodriguez P.L., Quesada V., Piqueras P., Ponce M.R. & Micol J.L. 2006. Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 29: 2000–2008.
Chappell J., VonLanken C. & Vogell U. 1991. Elicitor-inducible 3-hydroxy-3-methylglutaryl coenzyme A reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures. Plant Physiol. 97: 693–698.
Chappell J., Wolf F., Proulx J., Cuellar R. & Saunders C. 1995. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 109: 1337–1343.
Chen D.H., Ye H.C. & Li G.F. 2000. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci. 155: 179–185.
Fulzele D.P., Heble M.R. & Rao P.S. 1995. Production of terpenoid from Artemisia annua L. plantlet cultures in bioreactor. J. Biotechnol. 40: 139–143.
Guo C., Liu C.Z., Ye H.C. & Li G.F. 2004. Effect of temperature on growth and artemisinin biosynthesis in hairy root cultures of Artemisia annua. Acta Botanica Boreali — Occidentalia Sinica 24: 1828–1831.
Kim S.H., Chang Y.J. & Kim S.U. 2008. Tissue specificity and developmental pattern of amorpha-4,11-diene synthase (ADS) proved by ADS promoter-driven GUS expression in the heterologous plant, Arabidopsis thaliana. Planta Med. 74: 188–193.
Liersch R., Soicke H., Stehr C. & Tullner H.U. 1986 Formation of artemisinin in Artemisia annua during one vegetation period. Planta Med. 52: 387–390.
Liu C.Z., Guo C., Wang Y.C. & Ouyang F. 2002. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem. 38: 581–585.
Liu C.Z., Zhao Y. & Wang Y.C. 2006 Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 71: 11–20.
Mercke P., Bengtsson M., Bouwmeester H.J., Posthumus M.A. & Brodelius P.E. 2000. Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch. Biochem. Biophys. 381: 173–180.
Nair M.S., Acton N., Klayman D.L., Kendrick K., Basile D.V. & Mante S. 1986. Production of artemisinin in tissue cultures of Artemisia annua. J. Nat. Prod. 49: 504–507.
Putalun W., Luealon W., De-Eknamkul W., Tanaka H. & Shoyama Y. 2007. Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol. Lett. 29: 1143–1146.
Qian Z.H., Gong K., Zhang L., Lv J.B., Jing F.Y., Wang Y.Y., Guan S.B., Wang G.F. & Tang K.X. 2007. A simple and efficient procedure to enhance artemisinin content in Artemisia annua L. by seeding to salinity stress. Afric. J. Biotechnol. 6: 1410–1413.
Qureshi M.I., Israr M., Abdin M.Z. & Iqbal M. 2005. Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ. Exp. Bot. 53: 185–193.
Ro D.K., Paradise E.M., Ouellet M., Fisher K.J., Newman K.L., Ndungu J.M., Ho K.A., Eachus R.A., Ham T.S., Kirby J., Chang M.C.Y., Withers S.T., Shiba Y., Sarpong R. & Keasling J.D. 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440: 940–943.
Smet I.D., Zhang H., Inze D. & Beeckman T. 2006. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 11: 434–439.
Smith J.I., Smart N.J., Kurz W.G. & Misawa M. 1987. Stimulation of indole alkaloid production in cell suspension cultures of Catharanthus roseus by abscisic acid. Planta Med. 53: 470–474.
Smith T.C., Weathers P.J. & Cheetham R.C. 1997. Effect of gibberellic acid on hair root cultures of Artemisia annua growth and artemisinin production. In Vitro Cell. Dev. Biol. Plant 33: 75–79.
Tawfiq N.K., Anderson L.A., Roberts M.F., Phillipson J.D., Bray D.H. & Warhurst D.C. 1989. Antiplasmodial activity of Artemisia annua plant cell cultures. Plant Cell Rep. 8: 425–428.
Teoh K.H., Polichuk D.R., Reed D.W., Nowak G. & Covello P.S. 2006. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 580: 1411–1416.
Verslues P.E. & Zhu J.K. 2005. Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc. Trans. 33: 375–379.
Wallaart T.E., Bouwmeester H.J., Hille J., Poppinga L. & Maijers N.C.A. 2001. Amorpha-4,11-diene systhase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisnin. Planta 212: 460–465.
Wang Y.C., Zhang H.X., Zhao B. & Yuan X.F. 2001. Improved growth of Artemisia annua L. hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 23: 1971–1973.
Weathers P.J., Bunk G. & Mccoy M.C. 2005. The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell. Dev. Biol.Plant 41: 47–53.
Whipkey A., Simon J.E., Charles D.J. & Janick J. 1992. In vitro production of artemisinin from Artemisia annua L. Phytother. Res. 1: 15–25.
Zhang Y.S., Ye H.C., Liu B.Y., Wang H & Li G.F. 2005. Exogenous GA and flowering induce the conversion of artemisinic acid to artemisinin in Artemisia annua plants. Russian J. Plant Physiol. 52: 58–62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, F., Zhang, L., Li, M. et al. Abscisic acid (ABA) treatment increases artemisinin content in Artemisia annua by enhancing the expression of genes in artemisinin biosynthetic pathway. Biologia 64, 319–323 (2009). https://doi.org/10.2478/s11756-009-0040-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-009-0040-8