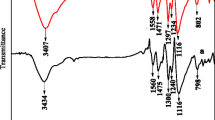
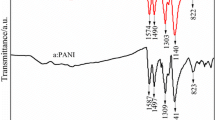
Abstract
Cobalt ion (Co2+)-doped polyaniline (PANI-Co), poly(N-methylaniline) (PNMA-Co), and poly(N-ethylaniline) (PNEA-Co) films were synthesised electrochemically on a pencil graphite electrode (PGE) and their electrochemical properties were investigated for supercapacitor applications. The polymer film-coated electrodes (PGE/PANI-Co, PGE/PNMA-Co, and PGE/PNEA-Co) thus obtained were characterised by scanning electron microscopy (SEM) and different electrochemical methods. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were employed in 0.1 M H2SO4 solution to calculate the specific capacitance (C S) values of the electrodes. The maximum C S of 192.94 F g−1, 139.83 F g−1, and 47.12 F g−1 were achieved for PGE/PANI-Co, PGE/PNMA-Co, and PGE/PNEA-Co at 1 mV s−1, respectively. On the other hand, the charge/discharge stability of the electrodes was analysed using the repeating chronopotentiometry (RCP) method. The RCP measurements indicate that the electrodes could be used as an electrode active material for low voltage supercapacitor applications.
Similar content being viewed by others
References
Abdelaziz, M. (2011). Cerium (III) doping effects on optical and thermal properties of PVA films. Physica B, 406, 1300–1307. DOI: 10.1016/j.physb.2011.01.021.
Arslan, A., & Hur, E. (2014). Electrochemical storage properties of polyaniline-, poly(N-methylaniline)-, and poly(N-ethylaniline)-coated pencil graphite electrodes. Chemical Papers, 68, 504–515. DOI: 10.2478/s11696-013-0475-9.
Cebeci, F. Ç., Geyik, H., Sezer, E., & Sarac, A. S. (2007). Synthesis, electrochemical characterization and impedance studies on novel thiophene-nonylbithiazole-thiophene comonomer. Journal of Electroanalytical Chemistry, 610, 113–121. DOI: 10.1016/j.jelechem.2007.07.012.
Chen, P. C., Shen, G., Shi, Y., Chen, H., & Zhou, C. (2010). Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano, 4, 4403–4411. DOI: 10.1021/nn100856y.
Davoglio, R. A., Biaggio, S. R., Bocchi, N., & Rocha-Filho, R. C. (2013). Flexible and high surface area composites of carbon fiber, polypyrrole, and poly(DMcT) for supercapacitor electrodes. Electrochimica Acta, 93, 93–100. DOI: 10.1016/j.electacta.2013.01.062.
Deshpande, P. P., Murali, M., Deshpande, P. P., Galphade, S., & More, A. (2013). Conducting poly(o-anisidine)-coated steel electrodes for supercapacitors. Chemical Papers, 67, 1066–1071. DOI: 10.2478/s11696-013-0317-9.
Dilgin, Y., Kızılkaya, B., Ertek, B., Eren, N., & Dilgin, D. G. (2012). Amperometric determination of sulfide based on its electrocatalytic oxidation at a pencil graphite electrode modified with quercetin. Talanta, 89, 490–495. DOI: 10.1016/j.talanta.2011.12.074.
Dubal, D. P., Kim, W. B., & Lokhande, C. D. (2012). Galvanostatically deposited Fe: MnO2 electrodes for supercapacitor application. Journal of Physics and Chemistry of Solids, 73, 18–24. DOI: 10.1016/j.jpcs.2011.09.005.
Dubal, D. P., & Lokhande, C. D. (2013). Significant improvement in the electrochemical performances of nano-nest like amorphous MnO2 electrodes due to Fe doping. Ceramics International, 39, 415–423. DOI: 10.1016/j.ceramint.2012.06.042.
Dubal, D. P., Patil, S. V., Gund, G. S., & Lokhande, C. D. (2013). Polyaniline-polypyrrole nanograined composite via electrostatic adsorption for high performance electrochemical supercapacitors. Journal of Alloys and Compounds, 552, 240–247. DOI: 10.1016/j.jallcom.2012.10.031.
Duran, B., Bereket, G., Turhan, M. C., & Virtanen, S. (2011). Poly(N-methyl aniline) thin films on copper: Synthesis, characterization and corrosion protection. Thin Solid Films, 519, 5868–5874. DOI: 10.1016/j.tsf.2011.02.084.
Fu, C., Zhou, H., Liu, R., Huang, Z., Chen, J., & Kuang, Y. (2012). Supercapacitor based on electropolymerized polythiophene and multi-walled carbon nanotubes composites. Materials Chemistry and Physics, 132, 596–600. DOI: 10.1016/j.matchemphys.2011.11.074.
Genies, E. M., & Tsintavis, C. (1985). Redox mechanism and electrochemical-behaviour of polyaniline deposits. Journal of Electroanalytical Chemistry, 195, 109–128. DOI: 10.1016/0022-0728(85)80009-7.
Genies, E. M., Syed, A. A., & Tsintavis, C. (1985). Electrochemical study of polyaniline in aqueous and organic medium. Redox and kinetic properties. Molecular Crystals and Liquid Crystals, 121, 181–186. DOI: 10.1080/00268948508074858.
Ghani, S. A., & Young, H. C. (2010). Conductive polymer based on polyaniline-eggshell powder (PANI-ESP) composites. Journal of Physical Science, 21, 81–97.
Gujar, T. P., Kim, W. Y., Puspitasari, I., Jung, K. D., & Joo, O. S. (2007). Electrochemically deposited nanograin ruthenium oxide as a pseudocapacitive electrode. International Journal of Electrochemical Science, 2, 666–673.
Gund, G. S., Dubal, D. P., Patil, B. H., Shinde, S. S., & Lokhande, C. D. (2013). Enhanced activity of chemically synthesized hybrid graphene oxide/Mn3O4 composite for high performance supercapacitors. Electrochimica Acta, 92, 205–215. DOI: 10.1016/j.electacta.2012.12.120.
Gupta, V., & Miura, N. (2005). Electrochemically deposited polyaniline nanowire’s network. A high-performance electrode material for redox supercapacitor. Electrochemical and Solid-State Letters, 8, A630–A632. DOI: 10.1149/1.2087207.
Gupta, V., & Miura, M. (2006). Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochimica Acta, 52, 1721–1726. DOI: 10.1016/j.electacta.2006.01.074.
Gurav, K. V., Patil, U. M., Shin, S. W., Agawane, G. L., Suryawanshi, M. P., Pawar, S. M., Patil, P. S., Lokhande, C. D., & Kim, J. H. (2013). Room temperature chemical synthesis of Cu(OH)2 thin films for supercapacitor application. Journal of Alloys and Compounds, 573, 27–31. DOI: 10.1016/j.jallcom.2013.03.193.
Heeger, A. J. (2002). Semiconducting and metallic polymers: the fourth generation of polymeric materials. Synthetic Metals, 125, 23–42. DOI: 10.1016/s0379-6779(01)00509-4.
Heli, H., Yadegari, H., & Jabbari, A. (2012). Graphene nanosheets-poly(o-aminophenol) nanocomposite for supercapacitor applications. Materials Chemistry and Physics, 134, 21–25. DOI: 10.1016/j.matchemphys.2012.02.065.
Hu, C. C., & Chu, C. H. (2000). Electrochemical and textural characterization of iridium-doped polyaniline films for electrochemical capacitors. Materials Chemistry and Physics, 65, 329–338. DOI: 10.1016/s0254-0584(00)00254-6.
Hu, Y., Zhu, H., Wang, J., & Chen, Z. (2011). Synthesis of layered birnessite-type manganese oxide thin films on plastic substrates by chemical bath deposition for flexible transparent supercapacitors. Journal of Alloys and Compdounds, 509, 10234–10240. DOI: 10.1016/j.jallcom.2011.08.080.
Izumi, C. M. S., Constantino, V. R. L., Ferreira, A. M. C., & Temperini, M. L. A. (2006). Spectroscopic characterization of polyaniline doped with transition metal salts. Synthetic Metals, 156, 654–663. DOI: 10.1016/j.synthmet.2005.12.023.
Jagadale, A. D., Kumbhar, V. S., Dhawale, D. S., & Lokhande, C. D. (2013). Performance evaluation of symmetric supercapacitor based on cobalt hydroxide [Co(OH)2] thin film electrodes. Electrochimica Acta, 98, 32–38. DOI: 10.1016/j.electacta.2013.02.094.
Jin, M., Han, G., Chang, Y., Zhao, H., & Zhang, H. (2011). Flexible electrodes based on polypyrrole/manganese dioxide/polypropylene fibrous membrane composite for supercapacitor. Electrochimica Acta, 56, 9838–9845. DOI: 10.1016/j.electacta.2011.08.079.
Justin, P., & Rao, G. R. (2010). CoS spheres for high-rate electrochemical capacitive energy storage application. International Journal of Hydrogen Energy, 35, 9709–9715. DOI: 10.1016/j.ijhydene.2010.06.036.
Kang, E. T., Neoh, K. G., & Tan, K. L. (1997). Photoelectron spectroscopy of conductive polymers. In H. S. Nalwa (Ed.), Handbook of organic conductive molecules and polymers (Vol. 3, Chapter 3, pp. 121–182). Chichester, UK: Wiley.
Li, Y., Zheng, J. L., Feng, J., & Jing, X. L. (2013) Polyaniline micro-/nanostructures: morphology control and formation mechanism exploration. Chemical Papers, 67, 876–890. DOI: 10.2478/s11696-013-0347-3.
MacDiarmid, A. G., Mammone, R. J., Kaner, R. B., Porter, S. J., Pethig, R., Heeger, A. J., & Rosseinsky, D. R. (1985). The concept of doping of conducting polymers: The role of reduction potentials [and discussion]. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 314, 3–15. DOI: 10.1098/rsta.1985.0004.
MacDiarmid, A. G. (2001). ”Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angewandte Chemie International Edition, 40, 2581–2590. DOI: 10.1002/1521-3773(20010716)40:14<2581::AID-ANIE2581>3.0.CO;2-2.
Mandić, Z., Kraljić Roković, M., & Pokupčić, T. (2009). Polyaniline as cathodic material for electrochemical energy sources: The role of morphology. Electrochimica Acta, 54, 2941–2950. DOI: 10.1016/j.electacta.2008.11.002.
Martinez, S., Valek, L., Petrović, Ž., Metikoš-Huković, M., & Piljac, J. (2005). Catechin antioxidant action at various pH studied by cyclic voltammetry and PM3 semi-empirical calculations. Journal of Electroanalytical Chemistry, 584, 92–99. DOI: 10.1016/j.jelechem.2005.07.015.
Mastragostino, M., Arbizzani, C., & Soavi, F. (2001). Polymerbased supercapacitors. Journal of Power Sources, 97–98, 812–815. DOI: 10.1016/s0378-7753(01)00613-9.
Mohamed, R. I., & Gadou, A. M. (2000). AC-conductivity and dielectric properties of γ-irradiated PVA films doped with Mn2+ ions. Egyptian Journal of Solids, 23, 277–286.
Patil, U. M., Salunkhe, R. R., Gurav, K. V., & Lokhande, C. D. (2008). Chemically deposited nanocrystalline NiO thin films for supercapacitor application. Applied Surface Science, 255, 2603–2607. DOI: 10.1016/j.apsusc.2008.07.192.
Patil, U. M., Kulkarni, S. B., Jamadade, V. S., & Lokhande, C. D. (2011). Chemically synthesized hydrous RuO2 thin films for supercapacitor application. Journal of Alloys and Compounds, 509, 1677–1682. DOI: 10.1016/j.jallcom.2010.09.133.
Pournaghi-Azar, M. H., & Habibi, B. (2007). Electropolymerization of aniline in acid media on the bare and chemically pre-treated aluminum electrodes: A comparative characterization of the polyaniline deposited electrodes. Electrochimica Acta, 52, 4222–4230. DOI: 10.1016/j.electacta.2006.11.050.
Ruffien-Ciszak, A., Gros, P., Comtat, M., Schmitt, A. M., Questel, E., Casas, C., & Redoules, D. (2006). Exploration of the global antioxidant capacity of the stratum corneum by cyclic voltammetry. Journal of Pharmaceutical and Biomedical Analysis, 40, 162–167. DOI: 10.1016/j.jpba.2005.05.035.
Shadi, L., Gheybi, H., Entezami, A. A., & Safa, K. D. (2012). Synthesis and characterization of N- and O-alkylated poly[aniline-co-N-(2-hydroxyethyl) aniline]. Journal of Applied Polymer Science, 124, 2118–2126. DOI: 10.1002/app.35218.
Sharma, R. K., & Zhai, L. (2009). Multiwall carbon nanotube supported poly(3,4-ethylenedioxythiophene)/manganese oxide nano-composite electrode for super-capacitors. Electrochimica Acta, 54, 7148–7155. DOI: 10.1016/j.electacta.2009.07.048.
Wang, Q., Li, J. L., Gao, F., Li, W. S., Wu, K. Z., & Wang, X. D. (2008). Activated carbon coated with polyaniline as an electrode material in supercapacitors. New Carbon Materials, 23, 275–280. DOI: 10.1016/s1872-5805(08)60030-x.
Wang, Q., Yan, J., Wang, Y., Ning, G., Fan, Z., Wei, T., Cheng, J., Zhang, M., & Jing, X. (2013). Template synthesis of hollow carbon spheres anchored on carbon nanotubes for high rate performance supercapacitors. Carbon, 52, 209–218. DOI: 10.1016/j.carbon.2012.09.022.
Wu, C., Wang, X., Ju, B., Zhang, X., Jiang, L., & Wu, H. (2012). Supercapacitive behaviors of activated mesocarbon microbeads coated with polyaniline. International Journal of Hydrogen Energy, 37, 14365–14372. DOI: 10.1016/j.ijhydene.2012.07.087.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hur, E., Arslan, A. Cobalt ion-doped polyaniline, poly(N-methylaniline), and poly(N-ethylaniline): electrosynthesis and characterisation using electrochemical methods in acidic solutions. Chem. Pap. 68, 1573–1583 (2014). https://doi.org/10.2478/s11696-014-0605-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0605-z