Abstract
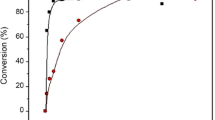
Imidazolium acetate ionic liquids show high efficiency in the degradation of polylactides (PLA): degradation degree of PLA can reach almost 100 % in imidazolium acetate ionic liquids at 170°C and 1 h under atmospheric pressure, while the degradation degree of PLA remains close to 0 % using neutral 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) and acidic ionic liquids at the same reaction conditions. With the increase of both the amount of acetate ionic liquid and the reaction temperature, the degradation degree of PLA increases. The structure of ionic liquids affects the degradation behavior of PLA: for cations, the proton from the C-2 position on the imidazolium ring is involved in the degradation of PLA; the degradation of PLA increases with the increase of the alkyl side-chain length of imidazolium cations; for anions, moderate basicity of the acetate ion contributes to the high activity of the imidazolium acetate ionic liquids in the degradation of PLA.
Similar content being viewed by others
References
de Jong, S. J., Arias, E. R., Rijkers, D. T. S., van Nostrum, C. F., Kettenes-van den Bosch, J. J., & Hennink, W. E. (2001). New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer, 42, 2795–2802. DOI: 10.1016/s0032-3861(00)00646-7.
He, X. L., Zhou, Q., Li, X. Y., Yang, P., van Kasteren, J. M. N., & Wang, Y. Z. (2012). Dechlorination of poly(vinyl chloride) by 1-butyl-3-methylimidazoliumhydroxide. Polymer Degradation and Stability, 97, 145–148. DOI: 10.1016/j.polymdegradstab.2011.11.005.
Höglund, A., Odelius, K., & Albertsson, A. C. (2013). Crucial differences in the hydrolytic degradation between industrial polylactide and laboratory-scale poly (L-lactide). ACS Applied Materials & Interfaces, 4, 2788–2793. DOI: 10.1021/am300438k.
Hollóczki, O., Gerhard, D., Massone, K., Szarvas, L., Németh, B., Veszprémi, T., & Nyulászi, L. (2010). Carbenes in ionic liquids. New Journal of Chemistry, 34, 3004–3009. DOI: 10.1039/c0nj00380h.
Huddleston, J. G., & Rogers, R. D. (1998). Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction. Chemical Communications, 1998, 1765–1766. DOI: 10.1039/a803999b.
Kamimura, A., & Yamamoto, S. (2007). An efficient method to depolymerize polyamide plastics: A new use of ionic liquids. Organic Letters, 9, 2533–2535. DOI: 10.1021/ol070886c.
Nampoothiri, K. M., Nair, N. R., & John, R. P. (2010). An overview of the recent developments in polylactide (PLA) research. Bioresource Technology, 101, 8493–8501. DOI: 10.1016/j.biortech.2010.05.092.
Park, K. I., & Xanthos, M. (2009). A study on the degradation of polylactic acid in the presence of phosphonium ionic liquids. Polymer Degradation and Stability, 94, 834–844. DOI: 10.1016/j.polymdegradstab.2009.01.030.
Rodríguez, H., Gurau, G., Holbrey, J. D., & Rogers, R. D. (2011). Reaction of elemental chalcogens with imidazolium acetates to yield imidazole-2-chalcogenones: direct evidence for ionic liquids as proto-carbenes. Chemical Communications, 47, 3222–3224. DOI: 10.1039/c0cc05223j.
Rydz, J., Adamus, G., Wolna-Stypka, K., Marcinkowski, A., Misiurska-Marczak, M., & Kowalczuk, M. M. (2013). Degradation of polylactide in paraffin and selected protic media. Polymer Degradation and Stability, 98, 316–324. DOI: 10.1016/j.polymdegradstab.2012.09.010.
Siracusa, V., Rocculi, P., Romani, S., & Rosa, M. D. (2008). Biodegradable polymers for food packaging: a review. Trends in Food Science & Technology, 19, 634–643. DOI: 10.1016/j.tifs.2008.07.003.
Wang, H., Liu, Y. Q., Li, Z. X., Zhang, X. P., Zhang, S. J., & Zhang, Y. Q. (2009). Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids. European Polymer Journal, 45, 1535–1544. DOI: 10.1016/j.eurpolymj.2009.01.025.
Xu, L., Crawford, K., & Gorman, C. B. (2011). Effects of temperature and pH on the degradation of poly(lactic acid) brushes. Macromolecules, 44, 4777–4782. DOI: 10.1021/ma2000948.
Zhao, T., Zhou, Q., He, X. L., Wei, S. D., Wang, L., van Kasteren, J. M. N., & Wang, Y. Z. (2010). A highly efficient approach for dehydrochlorinating polyvinyl chloride: catalysis by 1-butyl-3-methylimidazolium chloride. Green Chemistry, 12, 1062–1065. DOI: 10.1039/b927106f.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XY., Zhou, Q., Yang, KK. et al. Degradation of polylactide using basic ionic liquid imidazolium acetates. Chem. Pap. 68, 1375–1380 (2014). https://doi.org/10.2478/s11696-014-0560-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0560-8