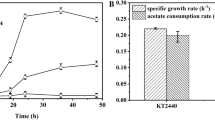
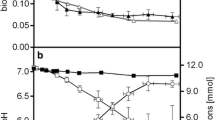
Abstract
Pseudomonas putida CGMCC3830 harboring nitrilase was used in isonicotinic acid production from 4-cyanopyridine. This nitrilase showed optimum activities towards 4-cyanopyridine at pH 7.5 and 45°C. The half-life of P. putida nitrilase was 93.3 h, 33.9 h, and 9.5 h at 30°C, 38°C, and 45°C, respectively. 4-Cyanopyridine (100 mM) was fully converted into isonicotinic acid within 20 min. The bench-scale production of isonicotinic acid was carried out using 3 mg of resting cells per mL in a 1 L system at 30°C and finally, 123 g L−1 of isonicotinic acid were obtained within 200 min without any by-products. The conversion reaction suffered from the product inhibition effect after the tenth feeding. The volumetric productivity was 36.9 g L−1 h−1. P. putida shows significant potential in nitrile hydrolysis for isonicotinic acid production. This paper is the first report on isonicotinic acid biosynthesis using Pseudomonas putida and it represents the highest isonicotinic acid production reported so far.
Similar content being viewed by others
References
Arai, M., Alavi, Y. I. H., Mendoza, J., Billker, O., & Sinden, R. E. (2004). Isonicotinic acid hydrazide: an antituberculosis drug inhibits malarial transmission in the mosquito gut. Experimental Parasitology, 106, 30–36. DOI: 10.1016/j.exppara.2004.01.002.
Banerjee, A., Kaul, P., & Banerjee, U. C. (2006). Enhancing the catalytic potential of nitrilase from Pseudomonas putida for stereoselective nitrile hydrolysis. Applied Microbiology and Biotechnology, 72, 77–87. DOI: 10.1007/s00253-005-0255-8.
Chaplin, J. A., Levin, M. D., Morgan, B., Farid, N., Li, J., Zhu, Z., McQuaid, J., Nicholson, L. W., Rand, C. A., & Burk, M. J. (2004). Chemoenzymatic approaches to the dynamic kinetic asymmetric synthesis of aromatic amino acids. Tetrahedron: Asymmetry, 15, 2793–2796. DOI: 10.1016/j.tetasy.2004.07.060.
Gong, J. S., Lu, Z. M., Li, H., Shi, J. S., Zhou, Z. M., & Xu, Z. H. (2012). Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microbial Cell Factories, 11, 142. DOI: 10.1186/1475-2859-11-142.
Kiziak, C., Conradt, D., Stolz, A., Mattes, R., & Klein, J. (2005). Nitrilase from Pseudomonas fluorescens EBC191: cloning and heterologous expression of the gene and biochemical characterization of the recombinant enzyme. Microbiology, 151, 3639–3648. DOI: 10.1099/mic.0.28246-0.
Kumar, V., & Bhalla, T. C. (2013). Transformation of p-hydroxybenzonitrile to p-hydroxybenzoic acid using nitrilase activity of Gordonia terrae. Biocatalysis and Biotransformation, 31, 42–48. DOI: 10.3109/10242422.2012.757761.
Layh, N., Parratt, J., & Willetts, A. (1998). Characterization and partial purification of an enantioselective arylacetonitrilase from Pseudomonas fluorescens DSM 7155. Journal of Molecular Catalysis B: Enzymatic, 5, 467–474. DOI: 10.1016/s1381-1177(98)00075-7.
Maksimova, Yu. G., Vasilyev, D. M., Ovechkina, G. V., Maksimov, A. Yu., & Demakov, V. A. (2013). Transformation of 2- and 4-cyanopyridines by free and immobilized cells of nitrile-hydrolyzing bacteria. Applied Biochemistry and Microbiology, 49, 347–351. DOI: 10.1134/s000368381304008x.
Malandra, A., Cantarella, M., Kaplan, O., Vejvoda, V., Uhnáková, B., Štěpáková, B., Kubáč, D., & Martínková, L. (2009). Continuous hydrolysis of 4-cyanopyridine by nitrilases from Fusarium solani O1 and Aspergillus niger K10. Applied Microbiology and Biotechnology, 85, 277–284. DOI: 10.1007/s00253-009-2073-x.
Martínková, L., & Křen, V. (2010). Biotransformations with nitrilases. Current Opinion in Chemical Biology, 14, 130–137. DOI: 10.1016/j.cbpa.2009.11.018.
Sharma, N., Sharma, M., & Bhalla, T. (2012). Nocardia globerula NHB-2 nitrilase catalysed biotransformation of 4-cyanopyridine to isonicotinic acid. AMB Express, 2, 25. DOI: 10.1186/2191-0855-2-25.
Vejvoda, V., Kaplan, O., Kubáč, D., Křen, V., & Martínková, L. (2006). Immobilization of fungal nitrilase and bacterial amidase — two enzymes working in accord. Biocatalysis and Biotransformation, 24, 414–418. DOI: 10.1080/10242420601033910.
Zhu, X. Y., Gong, J. S., Li, H., Lu, Z. M., Zhou, Z. M., Shi, J. S., & Xu, Z. H. (2013a). Screening, identification and culture optimization of a newly isolated aromatic nitrilaseproducing bacterium — Pseudomonas putida CGMCC3830. Chinese Journal of Biotechnology, in press.
Zhu, X. Y., Gong, J. S., Li, H., Lu, Z. M., Zhou, Z. M., Shi, J. S., & Xu, Z. H. (2013b). Characterization and functional cloning of an aromatic nitrilase from Pseudomonas putida CGMCC3830 with high conversion efficiency toward cyanopyridine. Journal of Molecular Catalysis B: Enzymatic, 97, 175–183. DOI: 10.1016/j.molcatb.2013.08.012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, XY., Gong, JS., Li, H. et al. Bench-scale biosynthesis of isonicotinic acid from 4-cyanopyridine by Pseudomonas putida . Chem. Pap. 68, 739–744 (2014). https://doi.org/10.2478/s11696-013-0521-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0521-7