Abstract
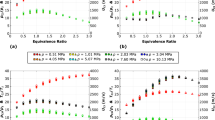
The kinetics and product distribution during the cracking of heptane in the presence of steam were investigated. The experiments were performed in a flow reactor under atmospheric pressure in a temperature range of 680–760°C with a mass ratio of steam to heptane of 3: 1. The overall decomposition of heptane is represented by a first-order reaction with activation energy of 249.1 kJ mol−1 and a frequency factor of 3.13 × 1013 s−1. The reaction products were analysed using gas chromatography, the main product being ethylene. The molecular reaction scheme, which consists of a primary reaction and 24 secondary reactions between primary products, was used for modelling the experimental product yields. The yields of ethylene and hydrogen were in good agreement; however the experimental yields of propylene were higher than the predicted yields.
Similar content being viewed by others
References
Albright, L. F., & Tsai, T. C. (1983). Importance of surface reactions units. In L. F. Albright, B. L. Crynes, & W. H. Corcoran (Eds.), Pyrolysis: Theory and industrial practice (pp. 233–254). New York, NY, USA: Academic Press.
Allara, D. L., & Shaw, R. (1980). A compilation of kinetic parameters for the thermal degradation of n-alkane molecules. Journal of Physical and Chemical Reference Data, 9, 523–560. DOI: 10.1063/1.555623.
Appleby, W. G., Aver, W. H., & Meerbott, W. K. (1947). Kinetics and mechanism of the thermal decomposition of n-heptane. Journal of the American Chemical Society, 69, 2279–2285. DOI: 10.1021/ja01202a012.
Aribike, D. S., & Susu, A. A. (1988a). Kinetics and mechanism of the thermal cracking of n-heptane. Thermochimica Acta, 127, 247–258. DOI: 10.1016/0040-6031(88)87501-4.
Aribike, D. S., & Susu, A. A. (1988b). Mechanistic modeling of the pyrolysis of n-heptane. Thermochimica Acta, 127, 259–273. DOI: 10.1016/0040-6031(88)87502-6.
Bajus, M., & Vesely, V. (1974). CS Patent No. 175812. Prague, Czechoslovakia: Czechoslovak Patent and Trademark Office.
Bajus, M., & Vesely, V. (1976). Hydrocarbon pyrolysis. I. Pyrolysis of individual n-alkanes. Ropa a Uhlie, 18, 126–135.
Bajus, M., Vesely, V., Leclercq, P. A., & Rijks, J. A. (1979). Steam cracking of hydrocarbons. 1. Pyrolysis of heptane. Industrial & Engineering Chemistry Product Research and Development, 18, 30–37. DOI: 10.1021/i360069a007.
Bajus, M. (1989). Sulfur compounds in hydrocarbon pyrolysis. Sulfur reports, 9, 25–71. DOI: 10.1080/01961778908047982.
Berreni, M., & Wang, M. H. (2011). Modelling and dynamic optimization of thermal cracking of propane for ethylene manufacturing. Computers & Chemical Engineering, 35, 2876–2885. DOI: 10.1016/j.compchemeng.2011.05.010.
Bounaceur, R., Warth, V., Marquaire, P. M., Scacchi, G., Dominé, F., Dessort, D., & Brevart, O. (2002). Modeling of hydrocarbons pyrolysis at low temperature. Automatic generation of free radicals mechanisms. Journal of Analytical and Applied Pyrolysis, 64, 103–122. DOI: 10.1016/s0165-2370(01)00173-5.
Chakraborty, J. P., & Kunzru, D. (2009). High pressure pyrolysis of n-heptane. Journal of Analytical and Applied Pyrolysis, 86, 44–52. DOI: 10.1016/j.jaap.2009.04.001.
Chakraborty, J. P., & Kunzru, D. (2012). High-pressure pyrolysis of n-heptane: Effect of initiators. Journal of Analytical and Applied Pyrolysis, 95, 48–55. DOI:10.1016/j.jaap.2012. 01.004.
Dente, M. E., & Ranzi, E. M. (1983). Mathematical modeling of hydrocarbon pyrolysis reactions. In L. F. Albright, B. L. Crynes, & W. H. Corcoran (Eds.), Pyrolysis: Theory and industrial practice (pp. 133–175). New York, NY, USA: Academic Press.
Ding, J. X., Zhang, L., & Han, K. L. (2011). Thermal rate constants of the pyrolysis of n-heptane. Combustion and Flame, 158, 2314–2324. DOI: 10.1016/j.combustflame.2011.04.015.
Ding, J. X., Zhang, L., Zhang, Y., & Han, K. (2013). A reactive molecular dynamics study of n-heptane pyrolysis at high temperature. The Journal of Physical Chemistry A, 117, 3266–3278. DOI: 10.1021/jp311498u.
Fabuss, B. M., Smith, J. O., & Satterfield, C. N. (1964). Thermal cracking of pure saturated hydrocarbons. In J. J. McKetta, Jr. (Ed.), Advances in petroleum chemistry and refining (Vol. 9, pp. 156–201). New York, NY, USA: Wiley.
Hájeková, E., & Bajus, M. (2005). Recycling of low-density polyethylene and polypropylene via copyrolysis of polyalkene oil/waxes with naphtha: product distribution and coke formation. Journal of Analytical and Applied Pyrolysis, 74, 270–281. DOI: 10.1016/j.jaap.2004.11.016.
Hájeková, E., Mlynková, B., Bajus, M., & Špodová, L. (2007). Copyrolysis of naphtha with polyalkene cracking products; the influence of polyalkene mixtures composition on product distribution. Journal of Analytical and Applied Pyrolysis, 79, 196–204. DOI: 10.1016/j.jaap.2006.12.022.
Hougen, O. A., & Watson, K. M. (1947). Chemical process principles (Vol. 3). New York, NY, USA: Wiley.
Jazayeri, S. M., & Karimzadeh, R. (2011). Experimental investigation of initial coke formation over stainless steel, chromium, and iron in thermal cracking of ethane with hydrogen sulfide as an additive. Energy & Fuels, 25, 4235–4247. DOI: 10.1021/ef2005173.
Kapur, S. (2005). ABB Lummus Global SRT® cracking technology for the production of ethylene. In R. A. Meyers (Ed.), Handbook of petrochemicals production processes (Chapter 6.1). New York, NY, USA: Mc Graw-Hill.
Karaba, A., Zamostny, P., Lederer, J., & Belohlav, Z. (2013). Generalized model of hydrocarbons pyrolysis using automated reactions network generation. Industrial & Engineering Chemistry Research, 52, 15407–15416. DOI: 10.1021/ie4006657.
Katta, V. R., Aggarwal, S. K., & Roquemore, W. M. (2012). Evaluation of chemical-kinetics models for n-heptane combustion using a multidimensional CFD code. Fuel, 93, 339–350. DOI: 10.1016/j.fuel.2011.10.035.
Kopinke, F. D., Zimmermann, G., & Ondruschka, B. (1987). Tendencies of aromatization in steam cracking of hydrocarbons. Industrial & Engineering Chemistry Research, 26, 2393–2397. DOI: 10.1021/ie00071a037.
Kossiakoff, A., & Rice, F. O. (1943). Thermal decomposition of hydrocarbons, resonance stabilization and isomerization of free radicals. Journal of the American Chemical Society, 65, 590–595. DOI: 10.1021/ja01244a028.
Murata, M., Saito, S., Amano, A., & Maeda, S. (1973). Prediction of initial product distributions from pyrolysis of normal paraffinic hydrocarbons. Journal of Chemical Engineering of Japan, 6, 252–258. DOI: 10.1252/jcej.6.252.
Pant, K. K., & Kunzru, D. (1996). Pyrolysis of n-heptane: kinetics and modeling. Journal of Analytical and Applied Pyrolysis, 36, 103–120. DOI: 10.1016/0165-2370(95)00925-6.
Reid, R. C., Prausnitz, J. M., & Poling, B. E. (1988). The properties of gases and liquids (4th ed.). New York, NY, USA: McGraw-Hill.
Reyniers, M. F. S. G., & Froment, G. F. (1995). Influence of metal surface and sulfur addition on coke deposition in the thermal cracking of hydrocarbons. Industrial & Engineering Chemistry Research, 34, 773–785. DOI: 10.1021/ie00042a009.
Rice, F. O., & Herzfeld, K. F. (1934). The thermal decomposition of organic compounds from the standpoint of free radicals. VI. The mechanism of some chain reactions. Journal of the American Chemical Society, 56, 284–289. DOI: 10.1021/ja01317a006.
Savage, P. E. (2000). Mechanisms and kinetics models for hydrocarbon pyrolysis. Journal of Analytical and Applied Pyrolysis, 54, 109–126. DOI: 10.1016/s0165-2370(99)00084-4.
Sundaram, K. M., & Froment, G. F. (1978). Modeling of thermal cracking kinetics. 3. Radical mechanisms for the pyrolysis of simple paraffins, olefins, and their mixtures. Industrial & Engineering Chemistry Fundamentals, 17, 174–182. DOI: 10.1021/i160067a006.
Yuan, T., Zhang, L. D., Zhou, Z. Y., Xie, M. F., Ye, L. L., & Qi, F. (2011). Pyrolysis of n-heptane: Experimental and theoretical study. The Journal of Physical Chemistry A, 115, 1593–1601. DOI: 10.1021/jp109640z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of professor Elemír Kossaczký
Rights and permissions
About this article
Cite this article
Olahová, N., Bajus, M., Hájeková, E. et al. Kinetics and modelling of heptane steam-cracking. Chem. Pap. 68, 1678–1689 (2014). https://doi.org/10.2478/s11696-013-0518-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0518-2