Abstract
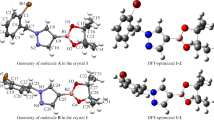
The structural characterisation of the molecule 1,4-bis[2-cyano-2-(o-pyridyl)ethenyl] benzene obtained through Knoevenagel condensation is reported. The single crystals, as light brown rods, were cultured from a chloroform solution using a slow evaporation method at ambient temperature. The compound crystallised in the monoclinic system belonging to the C2/c space group with a = 26.4556(9) Å, b = 3.73562(10) Å, c = 18.4230(6) Å, β = 109.841(4)° and the asymmetric unit comprising Z = 4. The structure is ordered and the molecules of the title compound exhibited a lattice with water molecules located at sites of inversion and two-fold axial symmetries. Thus, only halves of the molecules are symmetrically independent. The lattice is reported and contrasted with X-ray single-crystal diffraction and theoretical calculations of 1,4-bis(1-cyano-2-phenylethenyl)benzene. By using density functional theory (DFT) and second order Moller-Plesset (MP2) theoretical calculations, the ground state geometry in the whole molecule at the B3LYP/6-31+G(d,p), and MP2/6-31+G(d,p) theory levels, respectively, were optimised. The DFT calculations showed a quasi-planar structure of the molecule, whereas the wave function-based MP2 method afforded a non-planar optimised structure with significant torsion angles between the pyridine and phenyl rings.
Similar content being viewed by others
References
Agilent Technologies (2012). CrysAlisPro (Version 1.171.36.20) [computer program]. Santa Clara, CA, USA: Agilent Technologies.
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G., & Taylor, R. (1987). Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. Journal of the Chemical Society, Perkin Transactions 2, 1987, S1–S19. DOI: 10.1039/p298700000s1.
Andrade, S. G., Gonçalves, L. C. S., & Jorge, F. E. (2008). Scaling factors for fundamental vibrational frequencies and zeropoint energies obtained from HF, MP2, and DFT/DZP and TZP harmonic frequencies. Journal of Molecular Structure: THEOCHEM, 864, 20–25. DOI: 10.1016/j.theochem.2008.05.025.
Bartholomew, G. P., Bazan, G. C., Bu, X. H., & Lachicotte, R. J. (2000). Packing modes of distyrylbenzene derivatives. Chemistry of Materials, 12, 1422–1430. DOI: 10.1021/ cm991194o.
Bartocci, G., Mazzucato, U., Masetti, F., & Galiazzo, G. (1980). Excited state reactivity of aza aromatics. 9. Fluorescence and photoisomerization of planar and hindered styrylpyridines. The Journal of Physical Chemistry, 84, 847–851. DOI: 10.1021/j100445a010.
Bartocci, G., & Mazzucato, U. (1982). Conformational equilibria and photophysical behaviour of styrylpyridines; excitation energy effects in fluid and rigid solutions. Journal of Luminescence, 27, 163–175. DOI: 10.1016/0022-2313(82)90018-7.
Bauschlicher, C. W., & Langhoff, S. R. (1997). The calculation of accurate harmonic frequencies of large molecules: the polycyclic aromatic hydrocarbons, a case study. Spectrochimica Acta Part A, 53, 1225–1240. DOI: 10.1016/s1386-1425(97)00022-x.
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exchange. The Journal of Chemical Physics, 98, 5648–5652. DOI: 10.1063/1.464913.
Chapela, V. M., Percino, M. J., & Rodríguez-Barbarín, C. (2003). Crystal structure of 2,6-distyrylpyridine. Journal of Chemical Crystallography, 33, 77–83. DOI: 10.1023/a: 1023210422362.
Ditchfield, R., Hehre, W. J., & Pople, J. A. (1971). Self consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. The Journal of Chemical Physics, 54, 724–728. DOI: 10.1063/1.1674902.
Enkeimann, V. (1998). In K. Müllen, & G. Wegner (Eds.), Electronic materials: The oligomer approach. Weinheim, Germany: Wiley.
Friend, R. H., Gymer, R. W., Holmes, A. B., Burroughes, J. H., Marks, R. N., Taliani, C., Bradley, D. D. C., Dos Santos, D. A., Brédas, J. L., Löglund, M., & Salaneck, W. R. (1999). Electroluminiscence in conjugated polymers. Nature, 397, 121–128. DOI: 10.1038/16393.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr., J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, N. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2009). Gaussian 09 [computer program]. Wallingford, CT, USA: Gaussian.
Grimme, S. (2006). Semiempirical GGA-type density functional constructed with a long-range dispersion correction. Journal of Computational Chemistry, 27, 1787–1799. DOI: 10.1002/jcc.20495.
Greenham, N. C., Moratti, S. C., Bradley, D. D. C., Friend, R. H., & Holmes, A. B. (1993). Efficient light-emitting diodes based on polymers with high electron affinities. Nature, 365, 628–630. DOI: 10.1038/365628a0.
Head-Gordon, M., Pople, J. A., & Frisch, M. J. (1998). MP2 energy evaluation by direct methods. Chemical Physics Letters, 153, 503–506. DOI: 10.1016/0009-2614(88)85250-3.
Irngartinger, H., Lichtenthäler, J., & Herpich, R. (1994). Molecular structures and packing arrangements of five 2,5-bis(aryl-2-vinyl)-1,4-dimethoxybenzene derivatives. Structural Chemistry, 5, 283–289. DOI: 10.1007/bf02275501.
Kohn, W., & Sham, L. J. (1965). Self-consistent equations including exchange and correlation effects. Physical Review, 140, 1133–1138. DOI: 10.1103/physrev.140.a1133.
Lawson Daku, L. M., Linares, J., & Boillot, M. L. (2007). Ab initio static and molecular dynamics study of 4-styrylpyridine. ChemPhysChem, 8, 1402–1416. DOI: 10.1002/cphc.200700117.
Lee, C. T., Yang, W. T., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into functional of the electron density. Physical Reviews B, 37, 785–789. DOI: 10.1103/physrevb.37.785.
Li, X. C., Sirringhaus, H., Garnier, F., Holmes, A. B., Moratti, S. C., Feeder, N., Clegg, W., Teat, S. J., & Friend, R. H. (1998). A highly π-stacked organic semiconductor for thin film transistors based on fused thiophenes. Journal of the American Chemical Society, 120, 2206–2207. DOI: 10.1021/ja9735968.
Mackie, I. D., & DiLabio, G. A. (2008). Interactions in large, polyaromatic hydrocarbon dimers: Application of density functional theory with dispersion corrections. The Journal of Physical Chemistry A, 112, 10968–10976. DOI: 10.1021/jp806162t.
Marri, E., Galiazzo, G., Mazzucato, U., & Spalletti, A. (2005). Excited state properties of cross-conjugated 1,2- and 1,3-distyrylbenzene and some aza-analogues. Chemical Physics, 312, 205–211. DOI: 10.1016/j.chemphys.2004.11.038.
Melendez, F. J., Urzúa, O., Percino, M. J., & Chapela, V. M. (2010). A theoretical study on three conformational structures of 2,6-distyrylpyridine. International Journal of Quantum Chemistry, 110, 838–849. DOI: 10.1002/qua.22024.
Ośmiałowski, B., Kolehmainen, E., Nissinen, M., Krygowski, T. M., & Gawinecki, R. (2002). (1Z,3Z)-1,4-Di(pyridine-2-yl)buta-1,3-diene-2,3-diol: The planar highly conjugated symmetrical enediol with multiple intramolecular hydrogen bonds. The Journal of Organic Chemistry, 67, 3339–3345. DOI: 10.1021/jo016293b.
Percino, M. J., & Chapela, V. M. (1996). Poly(2,6-pyridinediylvinylene). In J. C. Salomone (Ed.), Polymeric materials encyclopedia (Vol. 9, pp. 6662–6670). New York, NY, USA: CRC Press. DOI: 0-8493-2470-x/96.
Percino, M. J., Chapela, V. M., Romero, S., Rodríguez-Barbarín, C., & Melendez-Bustamante, F. J. (2006). Synthesis of 1,2-dimethoxy-1,2-di(pyridin-2-yl)-1,2-ethanediol: Crystal and molecular structure determination. Journal of Chemical Crystallography, 36, 303–308. DOI: 10.1007/s10870-005-9064-2.
Percino, M. J., Chapela, V. M., Montiel, L. F., Pérez-Gutiérrez, E., & Maldonado, J. L. (2010). Spectroscopic characterization of halogen- and cyano-substituted pyridinevinylenes synthesized without catalyst or solvent. Chemical Papers, 64, 360–367. DOI: 10.2478/s11696-010-0012-z.
Percino, M. J., Chapela, V. M., Pérez-Gutiérrez, E., Cerón, M., & Soriano, G. (2011). Synthesis, optical, and spectroscopic characterisation of substituted 3-phenyl-2-arylacrylonitriles. Chemical Papers, 65, 42–51. DOI: 10.2478/s11696-010-0075-x.
Percino, M. J., Chapela, V. M., Cerón, M., Castro, M. E., Soriano-Moreno, G., Pérez-Gutiérrez, E., & Meléndez-Bustamante, F. (2012). Synthesis and characterization of conjugated pyridine-(N-diphenylamino) acrylonitrile derivatives: Photophysical properties. Journal of Materials Science Research, 1, 181–192. DOI: 10.5539/jmsr.v1n2p181.
Pérez-Gutiérrez, E., Percino, M. J., Chapela, V. M., Cerón, M., Maldonado, J. L., & Ramos-Ortiz, G. (2011). Synthesis, characterization, and photophysical properties of pyridinecarbazole acrylonitrile derivatives. Materials, 4, 562–574. DOI: 10.3390/ma4030562.
Renak, M. L., Bartholomew, G. P., Wang, S., Ricatto, P. J., Lachicotte, R. J., & Bazan, G. C. (1999). Fluorinated distyrylbenzene chromophores: Effect of fluorine regiochemistry on molecular properties and solid-state organization. Journal of the American Chemical Society, 121, 7787–7799. DOI: 10.1021/ja984440q.
Roothaan, C. C. J. (1951). New developments in molecular orbital theory. Reviews of Modern Physics, 23, 69–89. DOI: 10.1103/revmodphys.23.69.
Schlegel, H. B. (1982). Optimization of equilibrium geometries and transition structures. Journal of Computational Chemistry, 3, 214–218. DOI: 10.1002/jcc.540030212.
Shedrick, G. M. (1997) SHELXTL97 and SHELXTL2008 [computer program]. Göttngen, Germany: University of Göttngen.
Shetty, A. S., Liu, E. B., Lachicotte, R. J., & Jenekhe, S. A. (1999). X-ray crystal structures and photophysical properties of new conjugated oligoquinolines. Chemistry of Materials, 11, 2292–2295. DOI: 10.1021/cm981121p.
Simon, S., Duran, M., & Dannenberg, J. J. (1996). How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? Journal of Chemical Physics, 105, 11024–11031. DOI: 10.1063/1.472902.
Thanthiriwatte, K. S., Hohenstein, E. G., Burns, L. A., & Sherrill, C. D. (2011). Assessment of the performance of DFT and DFT-D methods for describing distance dependence of hydrogen-bonded interactions. Journal of Chemical Theory and Computation, 7, 88–96. DOI: 10.1021/ct100469b.
van Hutten, P. F., Wildeman, J., Meetsma, A., & Hadziioannou, G. (1999). Molecular packing in unsubstituted semiconduction phenylenevinylene oligomer and polymer. Journal of the American Chemical Society, 121, 5910–5918. DOI: 10.1021/ja990934r.
Wadsworth, D. H., Schupp, O. E., Seus, E. J., & Ford, J. A. (1965). The stereochemistry of the phosphonate modification of the Wittig reaction. The Journal of Organic Chemistry, 30, 680–685. DOI: 10.1021/jo01014a005.
Wu, G., Jacobs, S., Lenstra, A. T. H., van Alsenoy, C., & Geise, H. J. (1996). 2,5-Dimethoxy-1,4-bis[2-(2,4-dimethoxyphenyl) ethenyl]benzene studied by quantum chemical calculations and single crystal X-ray diffraction. Journal of Computational Chemistry, 17, 1820–1835. DOI: 10.1002/(sici)1096-987x(199612).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Percino, M.J., Castro, M.E., Ceron, M. et al. X-ray molecular structure and theoretical study of 1,4-bis[2-cyano-2-(o-pyridyl)ethenyl]benzene. Chem. Pap. 68, 272–282 (2014). https://doi.org/10.2478/s11696-013-0434-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0434-5