Abstract
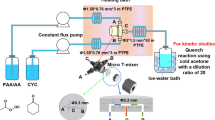
The reaction between caprolactam and ethanol was performed in near-critical water. The primary product (ethyl-6-aminohexanoate) was identified by GC-MS. The influences of the reaction temperature, residence time, initial ratio (reactant/water), pH, and additives on the yields of ethyl-6-aminohexanoate are discussed. The results showed that the yield of ethyl-6-aminohexanoate could be as high as 98 % with SnCl2 as an additive in near-critical water. At the same time, the reaction between caprolactam and ethanol was estimated by a lumped kinetic equation as a second-order reaction in near-critical water, and the activation energy was evaluated according to the Arrhenius equation under acidic and basic conditions. Based on the results, the reaction mechanism between caprolactam and ethanol in near-critical water is proposed.
Similar content being viewed by others
References
Abdulagatov, I. M., Bazaev, A. R., Bazaev, E. A., Saidakhmedova, M. B.,& Ramazanova, A. E. (1998). Volumetric properties of near-critical and supercritical water + pentane mixtures: Molar, excess, partial, and apparent volumes. Journal of Chemical & Engineering Data, 43, 451–458. DOI: 10.1021/je970287o.
Bicker, M., Endres, S., Ott, L.,& Vogel, H. (2005). Catalytical conversion of carbohydrates in subcritical water: A new chemical process for lactic acid production. Journal of Molecular Catalysis A: Chemical, 239, 151–157. DOI: 10.1016/j.molcata.2005.06.017.
Chang, Y. J., Wang, Z. Z., Luo, L. G.,& Dai, L. Y. (2012). Additive-assisted Rupe rearrangement of 1-ethynylcyclohexan-1-ol in near-critical water. Chemical Papers, 66, 33–38. DOI: 10.2478/s11696-011-0093-3.
Delaney, E. J., Wood, L. E.,& Klotz, I. M. (1982). Poly(ethylenimines) with alternative (alkylamino)pyridines as nucleophilic catalysts. Journal of the American Chemical Society, 104, 799–807. DOI: 10.1021/ja00367a025.
Díez Pascual, A. M., Compostizo, A., Crespo-Colín, A., & Rubio, R. G. (2007). Bulk and interfacial properties of a cationic micellar system near the critical point. Chemical Physics, 335, 124–132. DOI: 10.1016/j.chemphys.2007.04.004.
Duan, P. G., Li, S., Yang, Y., Wang, Z. Z.,& Dai, L. Y. (2009). Green medium for the hydrolysis of 5-cyanovaleramide. Chemical Engineering & Technology, 32, 771–777. DOI: 10.1002/ceat.200800607.
He, M. X., Feng, D. C., Zhu, F.,& Cai, Z. T. (2004). Alcoholysis of N-methyl-1,2-thiazetidine-1,1-dioxide: DFT study of water and alcohol effects. The Journal of Physical Chemistry A, 108, 7702–7708. DOI: 10.1021/jp048374s.
Kao, C. C., Ghita, O. R., Hallam, K. R., Heard, P. J.,& Evans, K. E. (2012). Mechanical studies of single glass fibres recycled from hydrolysis process using sub-critical water. Composites Part A: Applied Science and Manufacturing, 43, 398–427. DOI: 10.1016/j.compositesa.2011.11.011.
Kruse, A.,& Dinjus, E. (2007). Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. The Journal of Supercritical Fluids, 39, 362–380. DOI: 10.1016/j.supflu.2006.03.016.
Liu, C.,& Tobita, K. (2010). Hydraulic analysis of the water-cooled blanket based on the sub-critical water condition. Fusion Engineering and Design, 85, 979–982. DOI: 10.1016/j.fusengdes.2009.11.004.
Mi, J. L., Christensen, M., Tyrsted, C., Jensen, K.J., Hald, P.,& Iversen, B. B. (2010). Formation and growth of Bi2Te3 in biomolecule-assisted near-critical water: In situ synchrotron radiation study. The Journal of Physical Chemistry C, 114, 12133–12138. DOI: 10.1021/jp103858z.
Pacher, T., Raninger, A., Lorbeer, E., Brecker, L., But, P. P. H.,& Greger, H. (2010). Alcoholysis of naturally occurring imides: Misleading interpretation of antifungal activities. Journal of Natural Products, 73, 1389–1393. DOI: 10.1021/np1003092.
Rana, M. K.,& Chandra, A. (2012). Solvation structure of nanoscopic hydrophobic solutes in supercritical water: Results for varying thickness of hydrophobic walls, solute-solvent interaction and solvent density. Chemical Physics, 408, 28–35. DOI: 10.1016/j.chemphys.2012.09.008.
Riemenschneider, W., & Bolt, H. M. (2005). Esters, organic. In Ullmann’s encyclopedia of industrial chemistry. New York, NY, USA: Wiley. DOI: 10.1002/14356007.a09 565.pub2.
Szajna, E., Makowska-Grzyska, M. M., Wasden, C. C., Arif, A. M.,& Berreau, L. M. (2005). A deprotonated intermediate in the amide methanolysis reaction of an N4O-ligated mononuclear zinc complex. Inorganic Chemistry, 44, 7595–7605. DOI: 10.1021/ic050750f.
Vieitez, I., da Silva, C., Alckmin, I., Borges, G. R., Corazza, F. C., Oliveira, J. V., Grompone, M. A., & Jachmanián, I. (2010). Continuous catalyst-free methanolysis and ethanolysis of soybean oil under supercritical alcohol/water mixtures. Renewable Energy, 35, 1976–1981. DOI: 10.1016/j.renene.2010.01.027.
Watanabe, M., Sato, T., Ionmata, H., Smith, R. L., Jr., Arai, K., Kruse, A.,& Dinjus, E. (2004). Chemical reactions of C1 compounds in near-vritical and supercritical water. Chemical Reviews, 104, 5803–5822. DOI: 10.1021/cr020415y.
Watanabe, M., Iida, T., Aizawa, Y., Aida, T. M.,& Inomata, H. (2007). Acrolein synthesis from glycerol in hotcompressed water. Bioresource Technology, 98, 1285–1290. DOI: 10.1016/j.biortech.2006.05.007.
Yuksel, A., Sasaki, M.,& Goto, M. (2011). Complete degradation of Orange G by electrolysis in sub-critical water. Journal of Hazardous Materials, 190, 1058–1062. DOI: 10.1016/j.jhazmat.2011.02.083.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hou, Z.Q., Luo, L.G., Liu, C.Z. et al. Synthesis of ethyl-6-aminohexanoate from caprolactam and ethanol in near-critical water. Chem. Pap. 68, 164–169 (2014). https://doi.org/10.2478/s11696-013-0433-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0433-6