Abstract
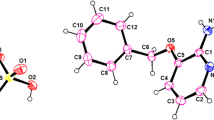
A new magnesium sulfate templated by 2-methylpiperazine, (C5H14N2)[Mg(H2O)6](SO4)2, was prepared by the slow evaporation method. The obtained crystals were investigated by the Raman and FTIR spectroscopy and crystallographically characterised by single-crystal X-ray diffraction. The compound crystallises in the monoclinic system, space group P21/n. Supramolecular network of this hybrid material consists of Mg2+ cations octahedrally coordinated by six water molecules, sulfate tetrahedra and protonated and disordered diamine linked together by two types of hydrogen bonds: OW—H…O and N—H…O. Dehydration of the title compound takes place in three steps. Thermal decomposition of the anhydrous phase consists in the loss of the organic moiety and one sulfate group leading to the formation of magnesium sulfate.
Similar content being viewed by others
References
Ben Ghozlen, M. H., Daoud, A., Paulus, H., & Pabst, I. (1994). Crystal structure of ethylenediammonium copper sulphate, [H3N(CH2)2NH3]Cu(SO4)2(H2O)4. Zeitschrift für Kristallographie — Crystalline Materials, 209, 383–383. DOI: 10.1524/zkri.1994.209.4.383.
Brandenburg, K., & Berndt, M. (2001). DIAMOND, Version 2.0. Bonn, Germany: Crystal Impact GbR.
Chaabouni, S., Kamoun, S., Daoud, A., & Jouini, T. (1996). Manganese ethylenediammonium bis(sulfate) tetrahydrate. Acta Crystallographica Section C, 52, 505–506. DOI: 10.1107/s0108270195011048.
Coppens, P. (1970). The evaluation of absorption and extinction in single-crystal structure analysis. In F. R. Ahmed, S. R. Hall, & C. P. Huber (Eds.), Crystallographic Computing: Proceedings of an International Summer School organized by The Commission on Crystallographic Computing of the International Union of Crystallography, August 4–11, 1969, Ottava, Canada (pp. 255–270). Copenhagen, Denmark: Munksgaard Publishers.
Farrugia, L. J. (1999). WinGX suite for small-molecule singlecrystal crystallography. Journal of Applied Crystallography, 32, 837–838. DOI: 10.1107/s0021889899006020.
Hajlaoui, F., Yahyaoui, S., Naïli, H., Mhiri, T., & Bataille, T. (2009). Synthesis, structural characterisation and thermal decomposition of a new organic-inorganic hybrid material (C5H14N2)[Cu(SO4)2(H2O)4] · H2O. Polyhedron, 28, 2113–2118. DOI: 10.1016/j.poly.2009.03.024.
Hajlaoui, F., Naïli, H., Yahyaoui, S., Turnbull, M. M., Mhiri, T., & Bataille, T. (2011). Synthesis, characterization and magnetic properties of four new organically templated metal sulfates [C5H14N2][MII(H2O)6](SO4)2, (MII = Mn, Fe, Co, Ni). Dalton Transactions, 40, 11613–11620. DOI: 10.1039/c1dt11030f.
Hajlaoui, F., Naïli, H., Yahyaoui, S., Norquist, A. J., Mhiri, T., & Bataille, T. (2012). Synthesis, crystal structures and thermal behaviour of organic-inorganic hybrids incorporating a chiral diamine. Journal of Organometallic Chemistry, 700, 110–116. DOI: 10.1016/j.jorganchem.2011.11.023.
Healy, P. C., Patrick, J. M., & White, A. H. (1984). Crystal structure of ethylenediammonium hexaaquanickel(II) sulfate: a variation on the Tutton salts. Australian Journal of Chemistry, 37, 1105–1109. DOI: 10.1071/ch9841105.
Held, P. (2003). Ethylenediammonium tetraaquabis(sulfato) iron(II). Acta Crystallographica Section E, 59, m197–m198. DOI: 10.1107/s1600536803004628.
Hill, C. L. (1998). Introduction: Polyoxometalatesmulticomponent molecular vehicles to probe fundamental issues and practical problems. Chemical Reviews, 98, 1–2. DOI: 10.1021/cr960395y.
Kagan, C. R., Mitzi, D. B., & Dimitrakopoulos, C. D. (1999). Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors. Science, 286, 945–947. DOI: 10.1126/science.286.5441.945.
Naïli, H., Rekik, W., Bataille, T., & Mhiri, T. (2006). Crystal structure, phase transition and thermal behaviour of dabcodiium hexaaquacopper(II) bis(sulfate), (C6H14N2) [Cu(H2O)6](SO4)2. Polyhedron, 25, 3543–3554. DOI: 10.1016/j.poly.2006.07.010.
Nonius BV (1998). Kappa CCD program software. Delft, The Netherlands: Nonius BV.
Otwinowski, Z., & Minor, W. (1997). Processing of X-ray diffraction data collected in oscillation mode. In C. W. Carter, Jr., & R. M. Sweet (Eds.), Macromolecular crystallography. Part A (Methods in Enzymology, Vol. 276, pp. 307–326). New York, NY, USA: Academic Press.
Rekik, W., Naïli, H., Bataille, T., Roisnel, T., & Mhiri, T. (2006a). Supramolecular networks of transition metal sulfates templated by piperazine. Inorganica Chimica Acta, 359, 3954–3962. DOI: 10.1016/j.ica.2006.05.030.
Rekik, W., Naïli, H., Bataille, T., & Mhiri, T. (2006b). Synthesis, crystal structure, phase transition and thermal behaviour of a new dabcodiium hexaaquanickel(II) bis(sulphate), (C6H14N2)[Ni(H2O)6](SO4)2. Journal of Organometallic Chemistry, 691, 4725–4732. DOI: 10.1016/j.jorganchem.2006.07.019.
Rekik, W., Naïli, H., Mhiri, T., & Bataille, T. (2008). [NH3(CH2)2NH3][Co(SO4)2(H2O)4]: Chemical preparation, crystal structure, thermal decomposition and magnetic properties. Materials Research Bulletin, 43, 2709–2718. DOI: 10.1016/j.materresbull.2007.10.024.
Rekik, W., Naïli, H., Mhiri, T., & Bataille, T. (2012). Three organically templated magnesium sulfates: Chemical preparation, hydrogen-bonded structures and thermal behavior. Solid State Sciences, 14, 1503–1511. DOI: 10.1016/j.solidstatesciences.2012.08.027.
Rujiwatra, A., & Limtrakul, J. (2005). Ethane-1,2-diaminium hexaaquazinc(II) sulfate. Acta Crystallographica Section E, 61, m1403–m1404. DOI: 10.1107/s1600536805019604.
Rull, F., Pastor, J. M., & De Saja, J. A. (1977). Des anomalies observées dans l’étude par effet Raman de La2(SO4)3 · 9H2O monocristallin en fonction de la temperature. Solid State Communications, 21, 221–224. DOI: 10.1016/0038-1098(77)90689-5. (in French)
Sheldrick, G. M. (1997). SHELXS-97 and SHELXL-97. Program for crystal structure resolution and analysis. Göttingen, Germany: University of Göttingen.
Yahyaoui, S., Rekik, W., Naïli, H., Mhiri, T., & Bataille, T. (2007). Synthesis, crystal structures, phase transition characterization and thermal decomposition of a new dabcodiium hexaaquairon(II) bis(sulfate): (C6H14N2)[Fe(H2O)6](SO4)2. Journal of Solid State Chemistry, 180, 3560–3570. DOI: 10.1016/j.jssc.2007.10.019.
Wang, A., Freeman, J. J., Jolliff, B. L., & Chou, I. M. (2006). Sulfates on Mars: A systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochimica et Cosmochimica Acta, 70, 6118–6135. DOI:10.1016/j.gca.2006.05.022.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, D.b., Rekik, W., Naïli, H. et al. A new organically templated magnesium sulfate: structure, spectroscopic analysis, and thermal behaviour. Chem. Pap. 68, 210–216 (2014). https://doi.org/10.2478/s11696-013-0432-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0432-7