Abstract
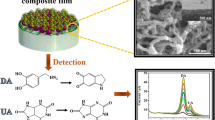
A new binuclear complex of copper2+, [LCu2+(CH3COO)2Cu2+L](CH3COO)2 where L is N,N-bis(phthalimide)ethylenediamine, was synthesised and characterised. The complex ion [LCu2+ (CH3COO)2Cu2+L]2+ was encapsulated into ZSM-5 zeolite and used to modify the surface of the glassy carbon electrode. This modified electrode, in a phosphate buffer solution at pH 7.0, exhibited an oxidation potential for dopamine (DA) and ascorbic acid (AA) at electrode potentials of 0.230 V and −0.090 V vs. Ag/AgCl respectively, a separation of 0.320 V. The electro-oxidation of DA or AA on the modified electrode is independent of each other. No interference was observed from Na+, K+, Cl−, SO 2−4 , Mg2+, Ca2+, Zn2+, Fe2+, and glucose. The detection limits obtained were 2.91 × 10−7 M for DA and 3.5 × 10−7 M for AA.
Similar content being viewed by others
References
Ali, S. R., Ma, Y. F., Parajuli, R. R., Balogan, Y., Lai, W. Y. C., & He, H. X. (2007). A nonoxidative sensor based on a self-doped polyanilline/carbon nanotube composite for sensitive and selective detection of the neurotransmitter dopamine. Analytical Chemistry, 79, 2583–2587. DOI: 10.1021/ac062068o.
Ardakani, M. M., Sheikh-Mohseni, M. A., Abdollahi-Alibaik, M., & Benvidi, A. (2012). Electrochemical sensors for simultaneous determination of norpinephrine, paracetamol and folic acid by a nonstructural mesoporous material. Sensors and Actuators B: Chemical, 171–172, 380–386. DOI: 10.1016/j.snb.2012.04.071.
Arrigoni, O., & De Tullio, M. C. (2002). Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta, 1569, 1–9. DOI: 10.1016/s0304-4165(01)00235-5.
Babaei, A., Zendehdel, M., Khaliljadeh, B., & Abnosi, M. (2010). A new sensor for simultanious determination of tyrosine and dopamine using iron(III) doped zeolite modified carbon paste electrode. Chinese Journal of Chemistry, 28, 1967–1972. DOI: 10.1002/cjoc.201090328.
Bustos, E. B., Jiménez, M.G. G., Díaz-Sánchez, B. R., Juaristi, E., Chapman, T. W., & Godínez, L. A. (2007). Glassy carbon electrodes modified with composites of starburst-PAMAM dendrimers containing metal nanoparticles for amperometric detection of dopamine in urine. Talanta, 72, 1586–1592. DOI: 10.1016/j.talanta.2007.02.017.
Cao, X. H., Zhang, L. X., Cai, W. P., & Li, Y. Q. (2010). Amperometric sensing of dopamine using a single-walled carbon nanotube covalently attached to a conical glass micropore electrode. Electrochemistry Communications, 12, 540–543. DOI: 10.1016/j.elecom.2010.01.038.
Chandra, U., Kumara Swamy, B. E., Gilbert, O., Shankar, S. S., Mahanthesha, K. R., & Sherigara, B. S. (2010). Electrocatalytic oxidation of dopamine at chemically modified carbon paste electrode with 2,4-dinitrophenyl hydrazine. International Journal of Electrochemical Science, 5, 1–9.
Cardero-Rando, M. M., Rodríguez, I. N., & de Cisneros, J. L. H. H. (1998). Voltammetric study of 2-methyl-4,6-dinitrophenol at a modified carbon paste electrode. Analytica Chimica Acta, 370, 231–238. DOI: 10.1016/s0003-2670(98)00262-1.
Damier, P., Hirsch, E. C., Agid, Y., & Graybiel, A. M. (1999). The substantia nigra of the human brain II. Patterns of loss of dopamine-containg neurons in Parkinson’s disease. Brain, 122, 1437–1448. DOI: 10.1093/brain/122.8.1437.
Dayton, M. A., Ewing, A. G., & Wightman, R. M. (1980). Response of microvoltammetric electrodes to homogeneous catalysis and slow heterogeneous charge-transfer reactions. Analytical Chemistry, 52, 2392–2396. DOI: 10.1021/ac50064 a035.
Dong, J. P., Zhou, X. J., Zhao, H. B., Xu, J. Q., & Sum, Y. B. (2011). Reagentless amperometric glucose biosensor based on the immobilization of glucose oxidase on a ferrocene@NaY zeolite composite. Microchimica Acta, 174, 281–288. DOI: 10.1007/s00604-011-0624-1.
Dursun, Z., & Nişli, G. (2004). Voltammetric behaviour of copper( I)oxide modified carbon paste electrode in the presence of cysteine and ascorbic acid. Talanta, 63, 873–878. DOI: 10.1016/j.talanta.2003.12.049.
Gopalan, A. I., Lee, K. P., Manesh, K. M., Santhosh, P., Kim, J. H., & Kang, J. S. (2007). Electrochemical determination of dopamine and ascorbic acid at a novel gold nanoparticles distributed poly(4-aminothiophenol) modified electrode. Talanta, 71, 1774–1781. DOI: 10.1016/j.talanta.2006.08.026.
Guirado, A., Zapata, A., & de Arellano, M. C. R. (1997). The reaction of phthalidylidene dichloride with primary amines. Synthesis and X-ray molecular structure of Nsubstituted phthalisoimides. Tetrahedron, 53, 5305–5324. DOI: 10.1016/s0040-4020(97)00194-4.
Kalita, B., & Talukdar, A. K. (2009). An efficient synthesis of nanocrystalline MFI zeolite using different silica sources: A green approach. Materials Research Bulletin, 44, 254–258. DOI: 10.1016/j.materresbull.2008.06.014.
Li, Y. X., Huang, X., Chen, Y. L., Wang, L., & Lin, X. Q. (2009). Simultaneous determination of dopamine and serotonin by use of covalent modification of 5-hydroxytryptophan on glassy carbon electrode. Microchimica Acta, 164, 107–112. DOI: 10.1007/s00604-008-0040-3.
Lin, X. H., Zhang, Y. F., Chen, W., & Wu, P. (2007). Electrocatalytic oxidation and determination of dopamine in the presence of ascorbic acid and uric acid at a poly(p-nitrobenzenazo resorcinol) modified glassy carbon electrode. Sensors and Actuators B: Chemical, 122, 309–314. DOI: 10.1016/j.snb.2006.06.004.
Liu, A. H., Honma, I., & Zhou, H. S. (2007). Simultaneous voltammetric detection of dopamine and uric acid at their physiological level in the presence of ascorbic acid using poly(acrylic acid)-multiwalled carbon-nanotube composite-covered glassy-carbon electrode. Biosenors and Bioelectronics, 23, 74–80. DOI: 10.1016/j.bios.2007.03.019.
Marko-Varga, G., Burested, E., Svensson, C. J., Emnéus, J., Gorton, L., Ruzgas, T., Lutz, M., & Unger, K. K. (1996). Effect of HY-zeolite on the performance of tyrocinase-modified carbon paste electrodes. Electroanalysis, 8, 1121–1126. DOI: 10.1002/elan.1140081209.
Martin, C. (1998). The Parkinson’s puzzle: New developments in our understanding of Parkinson’s disease have generated a number of promising new treatments for this disabling condition. Chemistry in Britain, 34(9), 40–42.
Mazloum-Ardakani, M., Akrami, Z., Kazemian, H., & Zare, H. R. (2009). Preconcentration and electroanalysis of copper at zeolite modified carbon paste electrode. International Journal of Electrochemical Science, 4, 308–319.
Molina, A., Villavicencio, C., & Fernández, L. (2009). Evaluation of a glassy carbon electrode modified with zeolite “A” in adsorption of 2-chlorophenol. Avances en Química, 4, 63–72. (in Spanish)
Neves, I., Freire, C., Zakhárov, A. N., de Castro, B., & Figueiredo, J. L. (1996). Zeolite-encapsulated copper (II) complexes with N3O2 Schiff bases: synthesis and characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 115, 249–256. DOI: 10.1016/0927-7757(96)03596-0.
Rajbongshi, J., Das, D. K., & Mazumdar, S. (2010). Direct electrochemistry of dinuclear CuA fragment from cytochrome c oxidase of Thermus thermophilus at surfactant modified glassy carbon electrode. Electrochimica Acta, 55, 4174–4179. DOI: 10.1016/j.electacta.2010.02.045.
Raoof, J. B., Ojani, R., & Rashid-Nadimi, S. (2005). Voltammetric determination of ascorbic acid and dopamine in the same sample at the surface of a carbon paste electrode modified with polypyrrole/ferrocyanide films. Electrochimica Acta, 50, 4694–4698. DOI: 10.1016/j.electacta.2005.03.002.
Rohani, T., & Taher, M. A. (2009). A new method for electrocatalytic oxidation of ascorbic acid at the Cu(II) zeolitemodified electrode. Talanta, 78, 743–747. DOI: 10.1016/j. talanta.2008.12.041.
Rohr, O., Sawaya, B. E., Lecestre, D., Aunis, D., & Schaeffer, E. (1999). Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-κB in cells of the immune system. Nucleic Acids Research, 27, 3291–3299. DOI: 10.1093/nar/27.16.3291.
Rolison, D. R. (1990). Zeolite-modified electrodes and electrodemodified zeolites. Chemical Reviews, 90, 867–878. DOI: 10.1021/cr00103a011.
Rover Júnior, L., Fernandes, J. C. B., de Oliviera-Neto, G., & Kubota, L. T. (2000). Development of a new FIA-potentiometric sensor for dopamine based on EVA-copper(II) ions. Journal of Electroanalytical Chemistry, 481, 34–41. DOI: 10.1016/s0022-0728(99)00474-x.
Senthikumar, S., & Saraswathi, R. (2009). Electrochemical sensing of cadmium and lead ions at zeolite — modified electrodes: Optimization and field measurements. Sensors and Actuators B: Chemical, 141, 65–75. DOI: 10.1016/j.snb.2009.05.029.
Shahrokhian, S., & Karimi, M. (2004). Voltammetric studies of a cobalt(II)-4-methylsalophen modified carbon-paste electrode and its application for the simultaneous determination of cysteine and ascorbic acid. Electrochimica Acta, 50, 77–84. DOI: 10.1016/j.electacta.2004.07.015.
Sotomayor, M. D. P. T., Tanaka, A. A., & Kubota, L. T. (2002). Development of an amperometric sensor for phenol compounds using a Nafion membrane doped with copper dipyridyl complex as a biomimetic catalyst. Journal of Electroanalytical Chemistry, 536, 71–81. DOI: 10.1016/s0022-0728(02)01205-6.
Suzuki, A., Ivandini, T. A., Yoshimi, K., Fujishima, A., Oyama, G., Nakazato, T., Hattori, N., Kitazawa, S., & Einaga, Y. (2007). Fabrication, characterization, and application of boron-doped diamond microelectrodes for in vivo dopamine detection. Analytical Chemistry, 79, 8608–8615. DOI: 10.1021/ac071519h.
Volkov, A., Tourillon, G., Lacaze, P. C., & Dubois, J. E. (1980). Electrochemical polymerization of aromatic amines: IR, XPS and PMT study of thin film formation on a Pt electrode. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 115, 279–291. DOI: 10.1016/s0022-0728(80)80332-9.
Walcarius, A. (1999). Zeolite modified electrodes in electroanalytical chemistry. Analytica Chimica Acta, 384, 1–16. DOI: 10.1016/s0003-2670(98)00849-6.
Wang, J., & Walcarius, A. (1996a). Zeolite containing oxidasebased carbon paste biosensor. Journal of Electroanalytical Chemistry, 404, 237–242. DOI: 10.1016/0022-0728(95)04357-8.
Wang, J., & Walcarius, A. (1996b). Zeolite-modified carbon paste elctrode for selective monitoring of dopamine. Journal of Electroanalytical Chemistry, 407, 183–187. DOI: 10.1016/0022-0728(95)04488-4.
Wang, M. G., Xu, X. G., & Gao, J. (2007). Voltammetric studies of a novel bicopper complex modified glassy carbon electrode for the simultaneous determination of dopamine and ascorbic acid. Journal of Applied Electrochemistry, 37, 705–710. DOI: 10.1007/s10800-007-9303-7.
Wang, G. F., Sun, J. G., Zhang, W., Jiao, S. F., & Fang, B. (2009). Simultaneous determination of dopamine, uric acid and ascorbic acid with LaFeO3 nanoparticles modified electrode. Microchimica Acta, 164, 357–362. DOI: 10.1007/s00604-008-0066-6.
Wightman, R. M., May, L. J., & Michael, A. C. (1988). Detection of dopamine dynamics in brain. Analytical Chemistry, 60, 769A–779A. DOI: 10.1021/ac00164a718.
Wu, W., Zhu, H. R., Fan, L. Z., Liu, D. F., Renneberg, R., & Yang, S. H. (2007). Sensitive dopamine recognition by boronic acid functionalized multiwalled carbon nanotubes. Chemical Communications, 23, 2345–2347. DOI: 10.1039/b701254c.
Xiao, Y. H., Guo, C. X., Li, C. M., Li, Y. B., Zhang, J., Xue, R. H., & Zhang, S. (2007). Highly sensitive and selective method to detect dopamine in the presence of ascorbic acid by a new polymeric composite film. Analytical Biochemistry, 371, 229–237. DOI: 10.1016/j.ab.2007.07.025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, D.K., Sarma, B. & Haloi, S. Synthesis and characterisation of a novel bi-nuclear copper2+ complex and its application as electrode-modifying agent for simultaneous voltammetric determination of dopamine and ascorbic acid. Chem. Pap. 68, 153–163 (2014). https://doi.org/10.2478/s11696-013-0431-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0431-8