Abstract
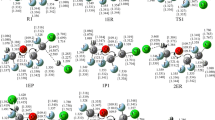
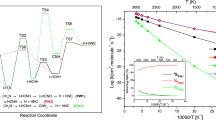
The singlet and triplet potential energy surfaces for the reaction of HS+ with the simplest primary amine, CH3NH2, were determined at the CCSD(T)/6-311+G(d,p) level using the B3LYP/6-311G(d,p) and QCISD/6-311G(d,p) geometries. All possible reaction channels were explored. The results show that three paths on the singlet potential energy surface and one path on the triplet potential energy surface are competitive. These four feasible paths provide products which are presented in the paper and they are consistent with previous experimental results. On the other hand, the stationary points involved in the most favourable path all lie below those of the reactant and thus the title reaction is expected to be rapid, which is also consistent with the experiment.
Similar content being viewed by others
References
Atroshchenko, Y. M., Shakhkel’dyan, I. E., Borbulevich, O. Y., Shchukin, A. N., Antipin, M. Y., & Khrustalev, V. N. (2005). Formation of isomeric 3-azabicyclo[3.3.1]nonanes in a reaction of 1-(2-hydroxyethoxy)-2,4-dinitrobenzene with sodium borohydride, formaldehyde, and methylamine. Russian Journal of Organic Chemistry, 41, 1683–1689. DOI: 10.1007/s11178-006-0019-7.
Baek, S. J., Choi, K. W., Choi, Y. S., & Kim, S. K. (2003a). Spectroscopy and dynamics of methylamine. I. Rotational and vibrational structures of CH3NH2 and CH3ND2 in states. The Journal of Chemical Physics, 118, 11026–11039. DOI: 10.1063/1.1575734.
Baek, S. J., Choi, K. W., Choi, Y. S., & Kim, S. K. (2003b). Spectroscopy and dynamics of methylamine. II. Rotational and vibrational structures of CH3NH2 and CH3ND2 in cationic D0 states. The Journal of Chemical Physics, 118, 11040–11047. DOI: 10.1063/1.1575735.
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648–5652. DOI: 10.1063/1.464913.
Cho, J., & Choi, C. H. (2011). Thermal decomposition mechanisms of methylamine, ethylamine, and 1-propylamine on Si(100)-2 × 1 surface. The Journal of Chemical Physics, 134, 194701. DOI: 10.1063/1.3589362.
Choi, M., Sukumar, N., Mathews, F. S., Liu, A., & Davidson, V. L. (2011). Proline 96 of the copper ligand loop of amicyanin regulates electron transfer from methylamine dehydrogenase by positioning other residues at the protein-protein interface. Biochemistry, 50, 1265–1273. DOI: 10.1021/bi101794y.
Conklin, D. J., Cowley, H. R., Wiechmann, R. J., Johnson, G. H., Trent, M. B., & Boor, P. J. (2004). Vasoactive effects of methylamine in isolated human blood vessels: role of semicarbazide-sensitive amine oxidase, formaldehyde, and hydrogen peroxide. American Journal of Physiology-Heart and Circulatory Physiology, 286, H667–H676. DOI: 10.1152/ajpheart.00690.2003.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J. A., Jr., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., & Pople, J. A. (2004). Gaussian 03, Revision D.02 [computer software]. Wallingford, CT, USA: Gaussian, Inc.
Gonzalez, C., & Schlegel, H. B. (1989). An improved algorithm for reaction path following. The Journal of Chemical Physics, 90, 2154–2161. DOI: 10.1063/1.456010.
Hamdani, S., Joly, D., Carpentier, R., & Tajmir-Riahi, H. A. (2009). The effect of methylamine on the solution structures of human and bovine serum albumins. Journal of Molecular Structure, 936, 80–86. DOI: 10.1016/j.molstruc.2009.07.019.
Ilyushin, V. V., Alekseev, E. A., Dyubko, S. F., Motiyenko, R. A., & Hougen, J. T. (2005). The rotational spectrum of the ground state of methylamine. Journal of Molecular Spectroscopy, 229, 170–187. DOI: 10.1016/j.jms.2004.08.022.
Irgibaeva, I. S. (2004). Determination of the parameters of multidimension vibration Hamiltonian of CH3NH2 from quantum chemical data. International Journal of Quantum Chemistry, 96, 210–218. DOI: 10.1002/qua.10648.
Jackson, D. M., Stibrich, N. J., Adams, N. G., & Babcock, L. M. (2005). A selected ion flow tube study of the reactions of a sequence of ions with amines. International Journal of Mass Spectrometry, 243, 115–120. DOI: 10.1016/j.ijms.2005.02.004.
Kerkeni, B., & Clary, D. C. (2007). Quantum scattering study of the abstraction reactions of H atoms from CH3NH2. Chemical Physics Letters, 438, 1–7. DOI: 10.1016/j.cplett.2007.02.046.
Kua, J., Krizner, H. E., & De Haan, D. O. (2011). Thermodynamics and kinetics of imidazole formation from glyoxal, methylamine, and formaldehyde: A computational study. The Journal of Physical Chemistry A, 115, 1667–1675. DOI: 10.1021/jp111527x.
Li, H., & Oshima, Y. (2005). Elementary reaction mechanism of methylamine oxidation in supercritical water. Industrial & Engineering Chemistry Research, 44, 8756–8764. DOI: 10.1021/ie0580506.
Lin, Z., Li, H., Luo, H., Zhang, Y., & Luo, W. (2011). Benzylamine and methylamine, substrates of semicarbazidesensitive amine oxidase, attenuate inflammatory response induced by lipopolysaccharide. International Immunopharmacology, 11, 1079–1089. DOI: 10.1016/j.intimp.2011.03.002.
Liu, P., Liu, J., Zhang, D., & Zhang, C. (2010). A comparative theoretical study of the reactivities of the Al+ and Cu+ ions toward methylamine and dimethylamine. International Journal of Quantum Chemistry, 110, 1583–1593. DOI: 10.1002/qua.22314.
Lu, X., Wei, S., Guo, W., & Wu, C. M. L. (2010). Mechanistic insight into the gas-phase reactions of methylamine with ground state Co+ (3F) and Ni+(2D). The Journal of Physical Chemistry A, 114, 12490–12497. DOI: 10.1021/jp106397g.
Lv, C. Q., Li, J., Ling, K. C., Shang, Z. F., & Wang, G. C. (2010). Methylamine decomposition on nickel surfaces: A density functional theory study. Surface Science, 604, 779–787. DOI: 10.1016/j.susc.2010.01.027.
Naganathappa, M., & Chaudhari, A. (2010). Absorption and vibrational spectra of methylamine and its ions using quantum chemical methods. Advances in Space Research, 45, 521–526. DOI: 10.1016/j.asr.2009.09.010.
Pople, J. A., Head-Gordon, M., & Raghavachari, K. (1987). Quadratic configuration interaction. A general technique for determining electron correlation energies. The Journal of Chemical Physics, 87, 5968–5975. DOI: 10.1063/1.453520.
Rudić, S., Murray, C., Harvey, J. N., & Orr-Ewing, A. J. (2003). The product branching and dynamics of the reaction of chlorine atoms with methylamine. Physical Chemistry Chemical Physics, 5, 1205–1212. DOI: 10.1039/b211626j.
Singh, P. C., Shen, L., Zhou, J., Schlegel, H. B., & Suits, A. G. (2010). Photodissociation dynamics of methylamine cation and its relevance to titan’s ionosphere. The Astrophysical Journal, 710, 112–116. DOI: 10.1088/0004-637x/710/1/112.
Smith, D., Adams, N. G., & Lindinger, W. (1981). Reactions of the HnS+ ions (n = 0 to 3) with several molecular gases at thermal energies. The Journal of Chemical Physics, 75, 3365–3370. DOI: 10.1063/1.442498.
Tian, W., Wang, W. L., Zhang, Y., & Wang, W. N. (2009). Direct dynamics study on the mechanism and the kinetics of the reaction of CH3NH2 with OH. International Journal of Quantum Chemistry, 109, 1566–1575. DOI: 10.1002/qua.22000.
Tiwary, A. S., & Mukherjee, A. K. (2009). Mechanism of the CH3NH2-HNO2 reaction: Ab initio DFT/TST study. Journal of Molecular Structure: THEOCHEM, 909, 57–65. DOI: 10.1016/j.theochem.2009.05.020.
Xiao, S., & Yu, P. H. (2009). A fluorometric high-performance liquid chromatography procedure for simultaneous determination of methylamine and aminoacetone in blood and tissues. Analytical Biochemistry, 384, 20–26. DOI: 10.1016/j.ab.2008.09.029.
Zeng, Y., Meng, L., Zheng, S., & Wang, D. (2003). B3LYP calculations of the potential energy surfaces of the thermal dissociations and the triplet ground state of pyrolysisproducts XN \(\left( {x^3 \sum ^ - } \right)\) for halogen azides XN3 (X: F, Cl, Br, I). Chemical Physics Letters, 378, 128–134. DOI: 10.1016/s0009-2614(03)01265-x.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, LL., Liu, HL., Tang, H. et al. Theoretical investigation on the reaction of HS+ with CH3NH2 . Chem. Pap. 68, 145–152 (2014). https://doi.org/10.2478/s11696-013-0412-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0412-y