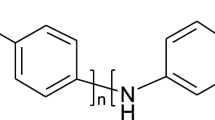
Abstract
Polyaniline (PANI) was synthesized by chemical oxidation of aniline in the presence of mixtures of water-soluble poly(sulfonic acids) of different nature. Under these conditions, the use of polyacid templates leads to the formation of interpolymer complexes of PANI and polyacid mixtures. The obtained PANI complexes were characterized by UV, visible, near IR, and Fourier transform infrared spectroscopy. It was shown that the rigidity of the polyacid backbone and the composition of a polyacid mixture affect the electronic structure of PANI complexes and the duration of the induction period of aniline oxidation. Domination of the more rigid-backbone template in the synthesis of PANI complexes with mixtures of the rigid- and flexible-backbone polyacids was observed. According to the viscometry and FTIR spectroscopic data, the reason of the domination is the existence of the intermolecular interaction between the polyacids in the mixture. In this case, duration of the induction period of aniline oxidation was between these values for pure polyacids.
Similar content being viewed by others
References
Boyer, M. I., Quillard, S., Rebourt, E., Louarn, G., Buisson, J. P., Monkman, A., & Lefrant, S. (1998). Vibrational analysis of polyaniline: A model compound approach. The Journal of Physical Chemistry B, 102, 7382–7392. DOI: 10.1021/jp972652o.
Boyer, M. L., Quillard, S., Louarn, G., Froyer, G., & Lefrant, S. (2000). Vibrational study of the FeCl3-doped dimer of polyaniline. A good model compound of emeraldine salt. The Journal of Physical Chemistry B, 104, 8952–8961. DOI: 10.1021/jp000946v.
Elliott, A. (1969). Infra-red spectra and structure of organic long-chain polymers. London, UK: Edward Arnold.
Epstein, A. J., Ginder, J. M., Zuo, F., Woo, H. S., Tanner, D. B., Richter, A. F., Angelopoulos, M., Huang, W. S., & MacDiarmid, A. G. (1987). Insulator-to-metal transition in polyaniline: Effect of protonation in emeraldine. Synthetic Metals, 21, 63–70. DOI: 10.1016/0379-6779(87)90067-1.
Fisher, L. W., Sochor, A. R., & Tan, J. S. (1977). Chain characteristics of poly(2-acrylamido-2-methylpropanesulfonate) polymers. 1. Light-Scattering and intrinsic-viscosity studies. Macromolecules, 10, 949–954. DOI: 10.1021/ma60059a012.
Gribkova, O. L., Nekrasov, A. A., Trchova, M., Ivanov, V. F., Sazikov, V. I., Razova, A. B., Tverskoy, V. A., & Vannikov, A. V. (2011). Chemical synthesis of polyaniline in the presence of poly(amidosulfonic acids) with different rigidity of the polymer chain. Polymer, 52, 2474–2484. DOI: 10.1016/j.polymer.2011.04.003.
Guseva, M. A., Isakova, A. A., Gribkova, O. L., Tverskoi, V. A., Ivanov, V. F., Vannikov, A. V., & Fedotov, Y. A. (2007). Matrix polymerization of aniline in the presence of polyamides containing sulfo acid groups. Polymer Science Series A, 49, 4–11. DOI: 10.1134/s0965545x07010026.
Hechavarría, L., Hu, H. L., & Rincon, M. E. (2003). Polyaniline-poly(2-acrylamido-2-methyl-1-propanosulfonic acid) composite thin films: structure and properties. Thin Solid Films, 441, 56–62. DOI: 10.1016/s0040-6090(03)00864-2.
Hu, H. L., Saniger, J. M., & Bañuelos, J. G. (1999). Thin films of polyaniline-polyacrylic acid composite by chemical bath deposition. Thin Solid Films, 347, 241–247. DOI: 10.1016/s0040-6090(99)00039-5.
Ivanov, V. F., Gribkova, O. L., Cheberyako, K. V., Nekrasov, A. A., Tverskoi, V. A., & Vannikov, A. V. (2004). Template synthesis of polyaniline in the precence of poly-(2-acrylamido-2-methyl-1-propanesulfonic acid). Russian Journal of Electrochemistry, 40, 299–304. DOI: 10.1023/b:ruel.0000019668.68527.cc.
Ivanov, V. F., Isakova, A. A., Gribkova, O. L., Nekrasov, A. A., Bogdanov, A. N., Vannikov, A. V., & Tverskoi, V. A. (2009). Peculiarities of polyaniline matrix synthesis in the presence of mixtures of different types of matrices and investigation of properties of formed interpolymer complexes. Protection of Metals and Physical Chemistry of Surfaces, 45, 548–552. DOI: 10.1134/s2070205109050086.
Ivanov, V. F., Gribkova, O. L., Omelchenko, O. D., Nekrasov, A. A., Tverskoy, V. A., & Vannikov, A. V. (2010). Effect of matrix domination in PANI interpolymer complexes with polyamidosulfonic acids. Journal of Solid State Electrochemistry, 14, 2011–2019. DOI: 10.1007/s10008-010-1049-1.
Kang, E. T., Neoh, K. G., & Tan, K. L. (1998). Polyaniline: A polymer with many interesting intrinsic redox states. Progress in Polymer Science, 23, 277–324. DOI: 10.1016/s0079-6700(97)00030-0.
Kirsh, Y. E., Fedotov, Y. A, Iudina, N. N., Artemov, D. Yu., Yanul’, N. A., & Nekrasova, T. N. (1991). On polyelectrolyte properties of sulfo-containing polyamides on the base of isoand terephthalic acids in aqueous solution. Polymer Science A, 33, 1127–1134.
Li, Y., Ying, B. Y., Hong, L. J., & Yang, M. J. (2010). Watersoluble polyaniline and its composite with poly(vinyl alcohol) for humidity sensing. Synthetic Metals, 160, 455–461. DOI: 10.1016/j.synthmet.2009.11.031.
Lin-Vien, D., Colthup, N. B., Fateley, W. G., & Grasselli, J. G. (1991). The handbook of infrared and Raman characteristic frequencies of organic molecules. San Diego, CA, USA: Academic Press.
MacDiarmid, A. G., & Epstein, A. J. (1994). The concept of secondary doping as applied to polyaniline. Synthetic Metals, 65, 103–116. DOI: 10.1016/0379-6779(94)90171-6.
Nekrasov, A. A., Ivanov, V. F., & Vannikov, A. V. (2000). Analysis of the structure of polyaniline absorption spectra based on spectroelectrochemical data. Journal of Electroanalytical Chemistry, 482, 11–17. DOI: 10.1016/s0022-0728(00)00005-x.
Omełchenko, O. D., Gribkova, O. L., Nekrasov, A. A., Tverskoi, V. A., Ivanov, V. F., & Vannikov, A. V. (2011). Nonadditive phenomena during polyaniline synthesis in the presence of mixtures of rigid-chain and flexible-chain polymer sulfoacids and their effect on properties of obtained interpolymer complexes. Protection of Metals and Physical Chemistry of Surfaces, 47, 503–511. DOI: 10.1134/s2070205111040149.
Pashkin, I. I., Tverskoi, V. A., & Pravednikov, A. N. (1987). Charge-transfer complexes of unit-type heterogeneous trinitrofluorenone-containing polymers. Polymer Science A, 29, 1631–1637.
Pavlov, G. M., Zaitseva, I. I., Gubarev, A. S., Gavrilova, I. I., & Panarin, E. F. (2006). Diffusion-viscometric analysis and conformational characteristics of sodium polystyrenesulfonate molecules. Russian Journal of Applied Chemistry, 79, 1490–1493. DOI: 10.1134/s1070427206090187.
Ping, Z. (1996). In situ FTIR-attenuated total reflection spectroscopic investigations on the base-acid transitions of polyaniline. Base-acid transition in the emeraldine form of polyaniline. Journal of the Chemical Society, Faraday Transactions, 92, 3063–3067. DOI: 10.1039/ft9969203063.
Quillard, S., Louarn, G., Buisson, J. P., Boyer, M., Lapkowski, M., Pron, A., & Lefrant, S. (1997). Vibrational spectroscopic studies of the isotope effects in polyaniline. Synthetic Metals, 84, 805–806. DOI: 10.1016/s0379-6779(96)04155-0.
Razova, A. B., Gribkova, O. L., Nekrasov, A. A., Ivanov, V. F., Tverskoi, V. A., & Vannikov, A. V. (2010). Influence of structure of polyacid on synthesis and properties of interpolymer polyaniline complexes. Protection of Metals and Physical Chemistry of Surfaces, 46, 540–545. DOI: 10.1134/s2070205110050060.
Socrates, G. (2001). Infrared and Raman characteristic group frequencies. New York, NY, USA: Wiley.
Stejskal, J., Sapurina, I., & Trchová, M. (2010). Polyaniline nanostructures and the role of aniline oligomers in their formation. Progress in Polymer Science, 35, 1420–1481. DOI: 10.1016/j.progpolymsci.2010.07.006.
Sun, L. F., Liu, H. B., Clark, R., & Yang, S. C. (1997). Double-strand polyaniline. Synthetic Metals, 84, 67–68. DOI: 10.1016/s0379-6779(96)03839-8.
Tarver, J., Yoo, J. E., Dennes, T. J., Schwartz, J., & Loo, Y. L. (2009). Polymer acid doped polyaniline is electrochemically stable beyond pH9. Chemistry of Materials, 21, 280–286. DOI: 10.1021/cm802314h.
Tengstedt, C., Crispin, A., Hsu, C. H., Zhang, C., Parker, I. D., Salaneck, W. R., & Fahlman, M. (2005). Study and comparison of conducting polymer hole injection layers in light emitting devices. Organic Electronics, 6, 21–33. DOI: 10.1016/j.orgel.2005.02.001.
Trchová, M., & Stejskal, J. (2011). Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical report). Pure and Applied Chemistry, 83, 1803–1817. DOI: 10.1351/pac-rep-10-02-01.
Trchová, M., Morávková, Z., Šeděnková, I., & Stejskal, J. (2012). Spectroscopy of thin polyaniline films deposited during chemical oxidation of aniline. Chemical Papers, 66, 415–445. DOI: 10.2478/s11696-012-0142-6.
Wessling, B. (2007). Conductive polymers as organic nanometals. In T. A. Skotheim, & J. R. Reynolds (Eds.), Handbook of conducting polymers: Conjugated polymers. Processing and applications (3rd ed., pp. 1–23). London, UK: Taylor & Francis.
Yang, S. M., Chen, W. M., & You, K. S. (1997). The properties of polyaniline-polyelectrolyte complexes. Synthetic Metals, 84, 77–78. DOI: 10.1016/s0379-6779(96)03844-1.
Yoo, J. E., Cross, J. L., Bucholz, T. L., Lee, K. S., Espe, M. P., & Loo, Y. L. (2007). Improving the electrical conductivity of polymer acid-doped polyaniline by controlling the template molecular weight. Journal of Materials Chemistry, 17, 1268–1275. DOI: 10.1039/b618521e.
Yoo, J. E., Bucholz, T. L., Jung, S., & Loo, Y. L. (2008). Narrowing the size distribution of the polymer acid improves PANI conductivity. Journal of Materials Chemistry, 18, 3129–3135. DOI: 10.1039/b802829j.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gribkova, O.L., Omelchenko, O.D., Trchová, M. et al. Preparation of polyaniline in the presence of polymeric sulfonic acids mixtures: the role of intermolecular interactions between polyacids. Chem. Pap. 67, 952–960 (2013). https://doi.org/10.2478/s11696-013-0384-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0384-y