Abstract
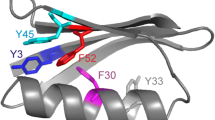
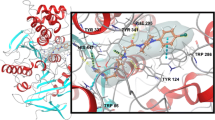
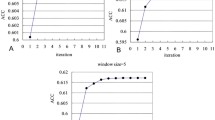
The presence of an unusually large number of aromatic residues in the active site gorge of acetylcholinesterase is a subject of great interest. Flexibility of these residues has been suspected to be a key player in controlling the ligand traversal in the gorge. This raises the question of whether the over-representation of aromatic residues in the gorge implies higher-than-normal flexibility of these residues. The current study suggests that it does not. Large changes in the hydrophobic cross-sectional area due to dihedral oscillations are probably the reason of their presence in the gorge.
Similar content being viewed by others
References
Birks, J., & Harvey, R. J. (2006). Donepezil for dementia due to Alzheimer’s disease. Cochrane Database of Systematic Reviews, 1, CD001190. DOI:10.1002/14651858.cd001190.pub2.
Davis, I.W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L.W., Arendall, W. B., Snoeyink, J., Richardson, J. S., & Richardson, D. C. (2007). MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Research, 35, W375–W383. DOI: 10.1093/nar/gkm216.
Dvir, H., Jiang, H. L., Wong, D. M., Harel, M., Chetrit, M., He, X. C., Jin, G. Y., Yu, G. L., Tang, X. C., Silman, I., Bai, D. L., & Sussman, J. L. (2002). X-ray structures of Torpedo californica acetylcholinesterase complexed with (+)-huperzine A and (−)-huperzine B: structural evidence for an active site rearrangement. Biochemistry, 41, 10810–10818. DOI:10.1021/bi020151.
Geula, C., & Mesulam, M. M. (1995). Cholinesterases and the pathology of Alzheimer disease. Alzheimer Disease & Associated Disorders, 9, 23–28. DOI: 10.1097/00002093-199501002-00005.
Gilson, M. K., Straatsma, T. P., McCammon, J. A., Ripoll, D. R., Faerman, C. H., Axelsen, P. H., Silman, I., & Sussman, J. L. (1994). Open “back door” in a molecular dynamics simulation of acetylcholinesterase. Science, 263, 1276–1278 DOI: 10.1126/science.8122110.
Harel, M., Sonoda, L. K., Silman, I., Sussman, J. L., & Rosenberry, T. L. (2008). Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetyl-cholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. Journal of the American Chemical Society, 130, 7856–7861. DOI: 10.1021/ja7109822.
Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics & Modelling, 14, 33–38. DOI: 10.1016/0263-7855(96)00018-5.
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W., & Klein, M. L. (1983). Comparison of simple potential functions for simulating liquid water. The Journal of Chemical Physics, 79, 926–935. DOI: 10.1063/1.445869.
Karplus, M., & McCammon, J. A. (2002). Molecular dynamics simulations of biomolecules. Nature Structural Biology, 9, 646–652. DOI: 10.1038/nsb0902-646.
Kryger, G., Silman, I., & Sussman, J. L. (1999). Structure of acetylcholinesterase complexed with E2020 (Aricept (R)): implications for the design of new anti-Alzheimer drugs. Structure with Folding & Design, 7, 297–307. DOI: 10.1016/s0969-2126(99)80040-9.
Luzar, A., & Chandler, D. (1996). Hydrogen-bond kinetics in liquid water. Nature, 379, 55–57. DOI: 10.1038/37905 5a0.
MacKerell, A. D., Bashford, D., Bellott, M., Dunbrack, R. L., Evanseck, J. D., Field, M. J., Fischer, S., Gao, J., Guo, H., Ha, S., Joseph-McCarthy, D., Kuchnir, L., Kuczera, K., Lau, F. T. K., Mattos, C., Michnick, S., Ngo, T., Nguyen, D. T., Prodhom, B., Reiher, W. E., Roux, B., Schlenkrich, M., Smith, J. C., Stote, R., Straub, J., Watanabe, M., Wiorkiewicz-Kuczera, J., Yin, D., & Karplus, M. (1998). Allatom empirical potential for molecular modeling and dynamics studies of proteins. Journal of Physical Chemistry B, 102, 3586–3616. DOI: 10.1021/jp973084f.
Mackerell, A. D., Feig, M., & Brooks, C. L. (2004). Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. Journal of Computational Chemistry, 25, 1400–1415. DOI:10.1002/jcc.20065.
Phillips, J. C., Braun, R., Wang, W., Gumbart, J., Tajkhorshid, E., Villa, E., Chipot, C., Skeel, R. D., Kalé, L., & Schulten, K. (2005). Scalable molecular dynamics with NAMD. Journal of Computational Chemistry, 26, 1781–1802. DOI:10.1002/jcc.20289.
Quinn, D. M. (1987). Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chemical Reviews, 87, 955–979. DOI: 10.1021/cr00081a005.
Shen, T. Y., Tai, K. H., Henchman, R. H., & McCammon, J. A. (2002). Molecular dynamics of acetylcholinesterase. Accounts of Chemical Research, 35, 332–340. DOI: 10.1021/ar010025i.
Sussman, J. L., Harel, M., Frolow, F., Oefner, C., Goldman, A., Toker, L., & Silman, I. (1991). Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science, 253, 872–879. DOI: 10.1126/science.1678899.
Tarek, M., & Tobias, D. J. (2002). Role of protein-water hydrogen bond dynamics in the protein dynamical transition. Physical Review Letters, 88, 138101. DOI: 10.1103/physrevlett.88.138101.
Wlodek, S. T., Clark, T. W., Scott, L. R., & McCammon, J. A. (1997). Molecular dynamics of acetylcholinesterase dimer complexed with tacrine. Journal of the American Chemical Society, 119, 9513–9522. DOI: 10.1021/ja971226d.
Wüthrich, K., & Wagner, G. (1978). Internal motion in globular proteins. Trends in Biochemical Sciences, 3, 227–230. DOI: 10.1016/s0968-0004(78)94607-8.
Xu, Y. C., Colletier, J. P., Weik, M., Jiang, H. L., Moult, J., Silman, I., & Sussman, J. L. (2008). Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophysical Journal, 95, 2500–2511. DOI: 10.1529/biophysj.108.129601.
Zhou, H. X., Wlodek, S. T., & McCammon, J. A. (1998). Conformation gating as a mechanism for enzyme specificity. Proceedings of the National Academy of Sciences of the United States of America, 95, 9280–9283.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
GhattyVenkataKrishna, P.K., Chavali, N. & Uberbacher, E.C. Flexibility of active-site gorge aromatic residues and non-gorge aromatic residues in acetylcholinesterase. Chem. Pap. 67, 677–681 (2013). https://doi.org/10.2478/s11696-013-0354-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0354-4