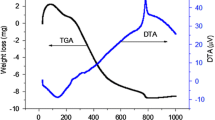
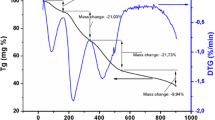
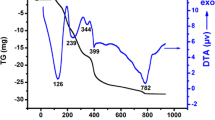
Abstract
A very simple, cost-effective, chloride- and alkali-free, carbonate co-precipitation synthesis in aqueous medium was applied in the preparation of perovskite-type lanthanum manganese oxide-based powders, i.e. La0.70Sr0.30MnO3−δ (LSM) and La0.75Sr0.25Cr0.5Mn0.5O3−δ (LSCrM). The precursors so obtained yielded nano-structured perovskite oxides when treated at 900°C and 800°C, respectively. The measured BET surface areas were in the low-end range for high temperature oxides (4 m2 g−1 and 10 m2 g−1) but the X-ray crystallite size was as low as 50 nm for LSCrM and 90 nm for LSM.
Similar content being viewed by others
References
Campanati, M., Fornasari, G., & Vaccari, A. (2003). Fundamentals in the preparation of heterogeneous catalysts. Catalysis Today, 77, 299–314. DOI: 10.1016/s0920-5861(02)00375-9.
Chou, Y. S., Stevenson, J.W., Armstrong, T. R., & Pederson, L. R. (2000). Mechanical properties of La1−x SrxCo0.2Fe0.8O3 mixed-conducting perovskites made by the combustion synthesis technique. Journal of the American Ceramic Society, 83, 1457–1464. DOI: 10.1111/j.1151-2916.2000.tb01410.x.
Colomer, M. T., Steele, B. C. H., & Kilner, J. A. (2002). Structural and electrochemical properties of the Sr0.8Ce0.1Fe0.7Co0.3O3−δ perovskite as cathode material for ITSOFCs. Solid State Ionics, 147, 41–48. DOI: 10.1016/s0167-2738(02)00002-4.
Cristiani, C., Bellotto, M., Forzatti, P., Lietti, L., Pasquon, I., & Villa, P. L. (1988). Preparation chemistry and phase transitions in the Zn-Mn-Cr system. Solid State Ionics, 26, 151–151. DOI: 10.1016/0167-2738(88)90068-9.
Cristiani, C., Zampori, L., Latorrata, S., Pelosato, R., Dotelli, G., & Ruffo, R. (2009). Carbonate coprecipitation synthesis of Sr- and Mg-doped LaGaO3. Materials Letters, 63, 1892–1894. DOI: 10.1016/j.matlet.2009.06.007.
Deleebeeck, L., Fournier, J. L., & Birss, V. (2010). Comparison of Sr-doped and Sr-free La1−x SrxMn0.5Cr0.5O3±δ SOFC anodes. Solid State Ionics, 181, 1229–1237. DOI: 10.1016/j.ssi.2010.05.027.
Garvie, R. C. (1965). The occurrence of metastable tetragonal zirconia as a crystallite size effect. The Journal of Physical Chemistry, 69, 1238–1243. DOI: 10.1021/j100888a024.
Grabowska, H., Miśta, W., Trawczyński, J., Wrzyszcz, J., & Zawadzki, M. (2001). Catalytic alkylation of phenol with methanol over zinc aluminate. Research on Chemical Intermediates, 27, 305–313 DOI: 10.1163/156856701300356527.
Groppi, G., Assandri, F., Bellotto, M., Cristiani, C., & Forzatti, P. (1995). The crystal-structure of Ba-β-alumina materials for high-temperature catalytic combustion. Journal of Solid State Chemistry, 114, 326–336. DOI: 10.1006/jssc.1995.1051.
Hsu, M. F., Wu, L. J., Wu, J. M., Shiu, Y. H., & Lin, K. F. (2006). Solid oxide fuel cell fabricated using all-perovskite materials. Electrochemical and Solid-State Letters, 9, A193–A195. DOI: 10.1149/1.2167929.
Huang, K., Feng, M., & Goodenough, J. B. (1996). Sol-gel synthesis of a new oxide-ion conductor Sr- and Mg-doped LaGaO3 perovskite. Journal of the American Ceramic Society, 79, 1100–1104. DOI: 10.1111/j.1151-2916.1996.tb08554.x.
Huang, K., Tichy, R. S., & Goodenough, J. B. (1998). Superior perovskite oxide-ion conductor; strontium- and magnesiumdoped LaGaO3: I. phase relationships and electrical properties. Journal of the American Ceramic Society, 81, 2565–2575. DOI: 10.1111/j.1151-2916.1998.tb02662.x.
Itoh, T., Shirasaki, S., Fujie, Y., Kitamura, N., Idemoto, Y., Osaka, K., Ofuchi, H., Hirayama, S., Honma, T., & Hirosawa, I. (2010). Study of charge density and crystal structure of (La0.75Sr0.25)MnO3.00 and (Ba0.5Sr0.5) (Co0.8Fe0.2)O2.33−δ at 500–900 K by in situ synchrotron X-ray diffraction. Journal of Alloys and Compounds, 491, 527–535. DOI: 10.1016/j.jallcom.2009.10.262.
Jiang, S. P. (2003). Issues on development of (La,Sr)MnO3 cathode for solid oxide fuel cells. Journal of Power Sources, 124, 390–402. DOI: 10.1016/s0378-7753(03)00814-0.
Kim, C. H., Qi, G., Dahlberg, K., & Li, W. (2010). Strontiumdoped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science, 327, 1624–1627. DOI: 10.1126/science.1184087.
Larson, A. C., & Von Dreele, R. B. (2004). General structure analysis system (GSAS). Report LAUR 86-748. Los Alamos, NM, USA: Los Alamos National Laboratory.
Liu, H., Hu, C., & Wang, Z. L. (2006). Composite-hydroxidemediated approach for the synthesis of nanostructures of complex functional-oxides. Nano Letters, 6, 1535–1540. DOI: 10.1021/nl061253e.
Pei, R. R., Chen, X., Suo, Y., Xiao, T., Ge, Q. Q., Yao, H. C., Wang, J. S., & Li, Z. J. (2012). Synthesis of La0.85Sr0.15Ga0.8Mg0.2O3−δ powder by carbonate co-precipitation combining with azeotropic-distillation process. Solid State Ionics, 219, 34–40. DOI: 10.1016/j.ssi.2012.05.022.
Pelosato, R., Cristiani, C., Dotelli, G., Latorrata, S., Ruffo, R., & Zampori, L. (2010). Co-precipitation in aqueous medium of La0.8Sr0.2Ga0.8Mg0.2O3−δ via inorganic precursors. Journal of Power Sources, 195, 8116–8123. DOI: 10.1016/j.jpowsour.2010.07.046.
Pelosato, R., Cristiani, C., Dotelli, G., Mariani, M., Donazzi, A., & Natali Sora, I. (2013). Co-precipitation synthesis of SOFC electrode materials. International Journal of Hydrogen Energy, 38, 65–71. DOI: 10.1016/j.ijhydene.2012.09.063.
Peña, M. A., & Fierro, J. L. G. (2001). Chemical structures and performance of perovskite oxides. Chemical Reviews, 101, 1981–2018. DOI: 10.1021/cr980129f.
Rietveld, H. M. (1969). A profile refinement method for nuclear and magnetic structures. Journal of Applied Crystallography, 2, 65–71. DOI: 10.1107/s0021889869006558.
Tao, S., & Irvine, J. T. S. (2003). A redox-stable efficient anode for solid-oxide fuel cells. Nature Materials, 2, 320–323. DOI: 10.1038/nmat871.
Tao, S., Irvine, J. T. S., & Kilner, J. A. (2005). An efficient solid oxide fuel cell based upon single-phase perovskites. Advanced Materials, 17, 1734–1737. DOI: 10.1002/adma.200402007.
Trimm, D. L. (1997). Deactivation and regeneration. In G. Ertl, H. Knözinger, & J. Weitkamp (Eds.), Handbook of heterogeneous catalysis (Vol. 1, Chapter 7, pp 1263–1282). Weinheim, Germany: Wiley-VCH.
Urban, J. J., Ouyang, L., Jo, M. H., Wang, D. S., & Park, H. (2004). Synthesis of single-crystalline La1−x BaxMnO3 nanocubes with adjustable doping levels. Nano Letters, 4, 1547–1550. DOI: 10.1021/nl049266k.
Xu, S., Moritomo, Y., Ohoyama, K., & Nakamura, A. (2003). Neutron structural analysis of La1−x SrxMnO3 — variation of one-electron bandwidth W with hole doping. Journal of the Physical Society of Japan, 72, 709–712. DOI: 10.1143/jpsj.72.709.
Zha, S., Tsang, P., Cheng, Z., & Liu, M. (2005). Electrical properties and sulfur tolerance of La0.75Sr0.25Cr1−x MnxO3 under anodic conditions. Journal of Solid State Chemistry, 178, 1844–1850. DOI: 10.1016/j.jssc.2005.03.027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cristiani, C., Dotelli, G., Mariani, M. et al. Synthesis of nanostructured perovskite powders via simple carbonate co-precipitation. Chem. Pap. 67, 526–531 (2013). https://doi.org/10.2478/s11696-013-0306-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0306-z