Abstract
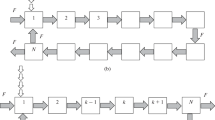
The study concentrates on the separation of aromatic hydrocarbons from aliphatic hydrocarbon mixtures using ionic liquids as a new alternative of extraction solvents. Influence of the phase equilibrium description accuracy on the separation equipment design using different thermodynamic models was investigated. As a model system, a heptane-toluene binary mixture was chosen, employing 1-ethyl-3-methylimidazolium ethyl sulfate (EMIES) ionic liquid as an extractive solvent. Liquid-liquid equilibrium (LLE) data of the ternary system were calculated using NRTL equations with different quality model parameters. Model 1 corresponds to the NRTL equation with the original binary parameters evaluated independently from the respective binary equilibrium data. Model 2 is represented by an NRTL equation extended by the ternary correction term (with the original binary parameters and ternary correction term parameters evaluated from the ternary tie-lines). Model 3, i.e. the NRTL equation with binary model parameters determined via ternary LLE data regression using ASPEN Plus, was taken from Meindersma et al. (2006). Continuous-flow liquidphase extraction was simulated considering a cascade of mixer-settler type extractors according to the Hunter-Nash scheme (Hunter & Nash, 1934). Based on the simulation results, for a preset separation efficiency criterium, different accuracies of the equilibrium description caused serious discrepancies in the separation equipment design, e.g. in the number of theoretical stages, solvent to feed ratio, and product purity.
Similar content being viewed by others
References
Ali, S. H., Lababidi, H. M. S., Merchant, S. Q., & Fahim, M. A. (2003). Extraction of aromatics from naphtha reformate using propylene carbonate. Fluid Phase Equilibria, 214, 25–38. DOI: 10.1016/s0378-3812(03)00323-6.
Arce, A., Earle, M. J., Katdare, S. P., Rodriguez, H., & Seddon, K. R. (2007). Phase equilibria of mixtures of mutually immiscible ionic liquids. Fluid Phase Equilibria, 261, 427–433. DOI: 10.1016/j.fluid.2007.06.017.
Aznar, M. (2007). Correlation of (liquid + liquid) equilibrium of systems including ionic liquids. Brazilian Journal of Chemical Engineering, 24, 143–149. DOI: 10.1590/s0104-66322007000100013.
Bendova, M., & Wagner, Z. (2009). Thermodynamic description of liquid-liquid equilibria in systems 1-ethyl-3-methylimidazolium ethylsulfate + C7-hydrocarbons by polymer-solution models. Fluid Phase Equilibria, 284, 80–85. DOI: 10.1016/j.fluid.2009.06.014.
Blažek, J., & Rabl, V. (2006). Základy zpracování a využití ropy (2nd ed.). Prague, Czech Republic: VŠCHT.
Chan, C., & Song, Y. H. (2004). Generalized electrolyte-NRTL model for mixed-solvent electrolyte systems. AIChE Journal, 50, 1928–1941. DOI: 10.1002/aic.10151.
Chen, C. C., Britt, H. I., Boston, J. F., & Evans, L. B. (1982). Local composition model for excess Gibbs energy of electrolyte systems. Part I: Single solvent, single completely dissociated electrolyte system. AIChE Journal, 28, 588–596. DOI: 10.1002/aic.690280410.
Choi, Y. J., Cho, K. W., Cho, B. W., & Yeo, Y. K. (2002). Optimization of the sulfolane extraction plant based on modeling and simulation. Industrial & Engineering Chemistry Research, 41, 5504–5509. DOI: 10.1021/ie010435a.
Domańska, U., Laskowska, M., & Marciniak, A. (2008). Phase equilibria of (1-ethyl-3-methylimidazolium ethylsulfate + hydrocarbon, + ketone, and + ether) binary systems. Journal of Chemical & Engineering Data, 53, 498–502. DOI: 10.1021/je700591h.
Garcia, J., Torrecilla, J. S., Fernandez, A., Oliet, M., & Rodriguez, F. (2010). (Liquid + liquid) equilibria in the binary systems (aliphatic, or aromatic hydrocarbons + 1-ethyl-3-methylimidazolium ethylsulfate, or 1-butyl-3-methylimidazolium methylsulfate ionic liquids). The Journal of Chemical Thermodynamics, 42, 144–150. DOI: 10.1016/j.jct.2009.07.023.
Gmehling, J., & Krummen, M. (2003). German Patent DE101 54052 DE. Munich, Germany: German Patent and Trade Mark Office.
Gonzalez, E. J., Calvar, N., Gomez, E., & Dominguez, A. (2010). Separation of benzene from linear alkanes (C6-C9) using 1-ethyl-3-methylimidazolium ethylsulfate at T = 298.15 K. Journal of Chemical & Engineering Data, 55, 3422–3427. DOI: 10.1021/je1001544.
Hansmeier, A. R., Jongsmans, M., Meindersma, G. W., & de Haan, A. B. (2010). LLE data for the ionic liquid 3-methyl-N-butyl pyridinium dicyanamide with several aromatic and aliphatic hydrocarbons. The Journal of Chemical Thermodynamics, 42, 484–490. DOI: 10.1016/j.jct.2009.11.001.
Hanusek, J. (2005). Iontove kapaliny — nový směr v “zelene” chemii. Chemické Listy, 99, 263–294.
Hendricks, E., Kontogeorgis, G. M., Dohrn, R., de Hemptinne, J. C., Economou, I. G., Fele Žilnik, L., & Vesovic, V. (2010). Industrial requirements for thermodynamics and transport properties. Industrial & Engineering Chemistry Research, 49, 11131–11141. DOI: 10.1021/ie101231b.
Huddleston, J. G., & Rogers, R. D. (1998). Room temperature ionic liquids as novel media for’ clean’ liquid-liquid extraction. Chemical Communications, 1998, 1765–1766. DOI: 10.1039/a803999b.
Hunter, T. G., & Nash, A. W. (1934). The application of physico-chemical principles to the design of liquid-liquid contact equipment. Part II: Application of phase-rule graphical method. Journal of the Society of Chemical Industry, 53, 95T–102T. DOI: 10.1002/jctb.5000531407.
Krishna, R., Goswami, A. N., Nanoti, S. M., Rawat, B. S., Khanna, M. K., & Dobhal, J. (1987). Extraction of aromatics from 63–69°C naphtha fraction for food grade hexane production using sulfolane and NMP as solvents. Indian Journal of Chemical Technology, 25, 602–606.
Krummen, M., Wasserchied, P., & Gmehling, J. (2002). Measurement of activity coeffitients at infinite dilution in ionic liquids using the dilutor technique. Journal of Chemical & Engineering Data, 47, 1411–1417. DOI: 10.1021/je0200517.
Meindersma, G. W. (2005). From solvent development to pilot RDC evaluation: Extraction of aromatics from naphtha with ionic liquids. PhD. thesis, University of Twente, Enschede, The Netherlands.
Meindersma, G. W., Podt, A. J. G., & de Haan, A. B. (2006). Ternary liquid-liquid equilibria for mixtures of toluene + nheptane + an ionic liquid. Fluid Phase Equilibria, 247, 158–168. DOI: 10.1016/j.fluid.2006.07.002.
Meindersma, G. W., & de Haan, A. B. (2008). Conceptual process design for aromatic/aliphatic separation with ionic liquids. Chemical Engineering Research and Design, 86, 745–752. DOI: 10.1016/j.cherd.2008.02.016.
Pereiro, A. B., Deive, F. J., Esperança, J. M. S. S., & Rodriguez, A. (2010). Alkylsulfate-based ionic liquids to separate azeotropic mixtures. Fluid Phase Equilibria, 294, 49–53. DOI: 10.1016/j.fluid.2010.05.006.
Perreiro, A. B., Araujo, J. M. M., Esperança, J. M. M. S., Marrucho, I. M., & Rebelo, L. P. N. (2012). Ionic liquids in separations of azeotropic systems — A review. The Journal of Chemical Thermodynamics, 46, 2–28. DOI: 10.1016/j.jct.2011.05.026.
Renon, H., & Prausnitz, J. M. (1968). Local compositions in thermodynamic excess functions for liquid mixtures. AIChE Journal, 14, 135–144. DOI: 10.1002/aic.690140124.
Schnieder, D. F. (2004). Avoid sulfolane regeneration problems. Chemical Engineering Progress, 100(7), 34–39.
Seiler, M., Jork, C., Kavarnou, A., Arlt, W., & Hirsch, R. (2004). Separation of azeotropic mixtures using hyperbranched polymers or iononic liquids. AIChE Journal, 50, 2439–2454. DOI: 10.1002/aic.10249.
Simoni, L. D., Lin, Y. D., Brennecke, J. F., & Stadtherr, M. A. (2008). Modeling liquid-liquid equilibrium of ionic liquid systems with NRTL, electrolyte-NRTL, and UNIQUAC. Industrial & Engineering Chemistry Research, 47, 256–272. DOI: 10.1021/ie070956j.
Sorensen, J. M., & Arlt, W. (1980a). Liquid-liquid equilibrium data collection. (DECHEMA Chemistry data series, Vol. V, Part 2. Ternary systems). Frankfurt/Main, Germany: DECHEMA.
Sorensen, J. M., & Arlt, W. (1980b). Liquid-liquid equilibrium data collection. (DECHEMA Chemistry data series, Vol. V, Part 3. Ternary and quaternary systems). Frankfurt/Main, Germany: DECHEMA.
Steltenpohl, P., & Graczova, E. (2010). Application of extended NRTL equation for ternary liquid-liquid and vapor-liquid-liquid equilibria description. Chemical Papers, 64, 310–317. DOI: 10.2478/s11696-010-0006-x.
Sumartschenkowa, I. A., Verevkin, S. P., Vasiltsova, T. V., Bich, E., Heintz, A., Shevelyova, M. P., & Kabo, G. J. (2006). Experimental study of thermodynamic properties of mixtures containing ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate using gas-liquid chromatography and transpiration method. Journal of Chemical & Engineering Data, 51, 2138–2144. DOI: 10.1021/je0602723.
Surovy, J., Dojčansky, J., & Bafrncova, S. (1982). The calculation of ternary liquid-liquid (L-L) equilibrium data using a ternary correction to the excess Gibbs free energy. Collection of Czechoslovak Chemical Communications, 47, 2380–2392. DOI: 10.1135/cccc19822380.
Varma, N. R., Ramalingam, A., & Banerjee, T. (2011). Experiments, correlations and COSMO-RS predictions for the extraction of benzothiophene from n-hexane using imidazolium-based ionic liquids. Chemical Engineering Journal, 166, 30–39. DOI: 10.1016/j.cej.2010.09.015.
Wauquier, J. P. (2000). Petroleum refining 2: Separation processes. Paris, France: Èditions Technip.
Weissermel, K., & Arpe, H. J. (2003). Industrial organic chemistry (4th ed., pp. 313–336). Weinheim, Germany: Wiley-VCH.
Yorulmaz, Y., & Karpuczu, F. (1985). Sulfolane versus diethylene glycol in recovery of aromatics. Chemical Engineering Research and Design, 63, 184–190.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graczová, E., Steltenpohl, P., Šoltýs, M. et al. Design calculations of an extractor for aromatic and aliphatic hydrocarbons separation using ionic liquids. Chem. Pap. 67, 1548–1559 (2013). https://doi.org/10.2478/s11696-012-0289-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0289-1