Abstract
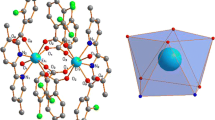
The thermal decomposition of lanthanide complexes, with a general formula: [LnL(NO3)2](NO3), where Ln = La, Pr, Nd, Sm, Gd, Tb, Dy, and Er; and L = bis-(salicyladehyde)-1,3-propylenediimine Schiff base ligand, was studied by thermogravimetric (TG) and derivative thermogravimetric (DTG) techniques. The TG and DTG data indicated that all complexes are thermostable up to 398 K. The thermal decomposition of all Ln(III) complexes was a two-stage process and the final residues were Ln2O3 (Ln = La, Nd, Sm, Gd, Dy, Er), Tb4O7, and Pr6 O11. The activation energies of thermal decomposition of the complexes were calculated from analysis of the TG-DTG curves using the Kissinger, Friedman, and Flynn-Well-Ozawa methods.
Similar content being viewed by others
References
Badea, M., Olar, R., Cristurean, E., Marinescu, D., Brezeanu, M., Balasoiu, M., & Segal, E. (1999). Thermal stability of some polynuclear coordination compounds in the systems Ln(III)—Co(II)—oxalate. Journal of Thermal Analysis and Calorimetry, 58, 103–111. DOI: 10.1023/a:1010131617489.
Emara, A. A. A., Saleh, A. A., & Adly, O. M. I. (2007). Spectroscopic investigations of new binuclear transition metal complexes of Schiff bases derived from 4,6-diacetylresorcinol and 3-amino-1-propanol or 1,3-diaminopropane. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 68, 592–604. DOI: 10.1016/j.saa.2006.12.034.
Ferenc, W., & Bocian, B. (2000). Thermal stability of 2,3,4-, 2,4,5- and 3,4,5-rimethoxybenzoates of light lanthanide. Journal of Thermal Analysis and Calorimetry, 60, 131–138. DOI: 10.1023/a:1010188905767.
Friedman, H. L. (1964). Kinetics of thermal degradation of charforming plastics from thermogravimetry. Application to a phenolic plastic. Journal of Polymer Science Part C: Polymer Symposia, 6, 183–195. DOI: 10.1002/polc.5070060121.
Hussein, G. A. M., Buttrey, D. J., DeSanto, P., Abd-Elgaber, A. A., Roshdy, H., & Myhoub, A. Y. Z. (2003). Formation and characterization of samarium oxide generated from different precursors. Thermochimica Acta, 402, 27–36. DOI: 10.1016/s0040-6031(02)00535-x.
Kumar, A. S., & Indrasenan, P. (2008). Thermal decomposition studies of lanthanide(III) complexes of EDTA. Asian Journal of Chemistry, 20, 5178–5186.
Kissinger, H. E. (1957). Reaction kinetics in differential thermal analysis. Analytical Chemistry, 29, 1702–1706. DOI: 10.1021/ac60131a045.
Li, Q., Li, T., & Wu, J. (2001). Luminescence of europium(III) and terbium(III) complexes incorporated in poly(vinyl pyrrolidone) matrix. The Journal of Physical Chemistry B, 105, 12293–12296. DOI: 10.1021/jp012922+.
Marques, R. N., Melios, C. B., & Ionashiro, M. (2002). Synthesis, characterisation and thermal behaviour of solid state compounds of 4-methylbenzylidenepyruvate with heavier trivalent lanthanides and yttrium(III). Thermochimica Acta, 395, 145–150. DOI: 10.1016/s0040-6031(02)00143-0.
Mary, N. L., & Parameswaran, G. (1991). Kinetics and mechanism of the thermal decomposition of Schiff base complexes of lanthanides by TG and DSC studies. Thermochimica Acta, 185, 345–353. DOI: 10.1016/0040-6031(91)80055-n.
Ozawa, T. (1965). A new method of analyzing thermogravimetric data. Bulletin of the Chemical Society of Japan, 38, 1881–1886. DOI: 10.1246/bcsj.38.1881.
Petoud, S., Cohen, S. M., Bünzli, J. C. G., & Raymond, K. N. (2003). Stable lanthanide luminescence agents highly emissive in aqueous solution: Multidentate 2-hydroxyisophthalamide complexes of Sm3+, Eu3+, Tb3+, Dy3+. Journal of the American Chemical Society, 125, 13324–13325. DOI: 10.1021/ja0379363.
Taha, Z. A., Ajlouni, A. M., Al-Hassan, K. A., Hijazi, A. K., & Faiq, A. B. (2011). Syntheses, characterization, biological activity and fluorescence properties of bis-(salicylaldehyde)-1,3-propylenediimine Schiff base ligand and its lanthanide complexes. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 81, 317–323. DOI: 10.1016/j.saa.2011.06.018.
Vicente, M., Bastida, R., Lodeiro, C., Macías, A., Parola, A. J., Valencia, L., & Spey, S. E. (2003). Metal complexes with a new N4O3 amine pendant-armed macrocyclic ligand: Synthesis, characterization, crystal structures, and fluorescence studies. Inorganic Chemistry, 42, 6768–6779. DOI: 10.1021/ic034245z.
Woods, M., Kovacs, Z., & Sherry, A. D. (2002). Targeted complexes of lanthanide(III) ions as therapeutic and diagnostic pharmaceuticals. Journal of Supramolecular Chemistry, 2, 1–15. DOI: 10.1016/s1472-7862(02)00072-2.
Yin, C. M., Liu, Z. R., Kong, Y. H., Wu, C. Y., Ren, D. H., Lü, Y. G., & Xue, H. F. (1992). Studies on the thermal behaviour and decomposition mechanism of complexes of rare earth(III) nitrates with 18-crown-6. Thermochimica Acta, 204, 251–260. DOI: 10.1016/0040-6031(92)85229-o.
Zheng, Y., Fu, L., Zhou, Y., Yu, J., Yu, Y., Wang, S., & Zhang, H. (2002). Electroluminescence based on a β-diketonate ternary samarium complex. Journal of Materials Chemistry, 12, 919–923. DOI: 10.1039/b110373c.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taha, Z.A., Ajlouni, A.M. & Al-Mustafa, J. Thermal decomposition of lanthanide(III) complexes of bis-(salicylaldehyde)-1,3-propylenediimine Schiff base ligand. Chem. Pap. 67, 194–201 (2013). https://doi.org/10.2478/s11696-012-0262-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0262-z