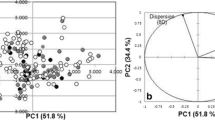
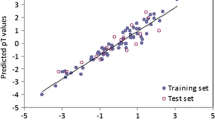
Abstract
Partition coefficients between environmental compartments are essential parameters in any predictive models on pollutants’ fate in various emission scenarios. When sufficient experimental data are not available, empirical algebraic models are capable of predicting the pollutant partitioning characteristics based on bulk physico-chemical properties or various molecular structural features. When the use of sophisticated rules based on detailed 2D–3D molecular descriptors is not available as a quick option, inexpensive, simple correlations based solely on octanol-1-ol (octanol)-water partition coefficients (K ow) are extensively employed. The present study investigates enhancing the adequacy of such hydrophobicity-based models by adding simple 1D descriptors, readily identifiable by inspecting the substance structure (i.e. the number of chlorine atoms bound to aromatic rings, or the number of aromatic 5- or 6-atom rings, etc.), in addition to the pollutant’s solubility in water. Exemplification is made for predicting the water-biota (fish)-sediment partition coefficients for chlorobenzenes (CBz).
Similar content being viewed by others
References
American Society for Testing and Materials (1984). Standard practice for conducting bioconcentration test with fishes and salt-water bi-valve mollusk. ASTM E-1022-84. West Conshohocken, PA, USA.
American Society for Testing and Materials (2001). Standard test methods for determining a sorption constant (Koc) for an organic chemical in soil and sediments. ASTM E-1195-01. West Conshohocken, PA, USA.
Axelman, J., Broman, D., Näf, C., & Pettersen, H. (1995). Compound dependence of the relationship log Kow and log BCFL. A comparison between chlorobenzenes (CBs) for rainbow trout and polycyclic aromatic hydrocarbons (PAHs) for Daphnia. Environmental Science and Pollution Research, 2, 33–36. DOI: 10.1007/bf02987509.
Bahadur, N. P., Shiu, W. Y., Boocock, D. G. B., & Mackay, D. (1997). Temperature dependence of octanol-water partition coefficient for selected chlorobenzenes. Journal of Chemical & Engineering Data, 42, 685–688. DOI: 10.1021/je970020p.
Barber, M. C. (2003). A review and comparison of models for predicting dynamic chemical bioconcentration in fish. Environmental Toxicology and Chemistry, 22, 1963–1992. DOI: 10.1897/02-468.
Bates, D. M., & Watts, D. G. (1988). Nonlinear regression analysis and its applications. New York, NY, USA: Wiley.
Chiriac, A., Ciubotaru, D., & Simon, Z. (1996). Relaţii cantitative structură cimică — activitate biologică (QSAR). Timişoara, Romania: Mirton Publ.
Djohan, D., Yu, Q., & Connell, D. W. (2005). Partition isotherms of chlorobenzenes in a sediment-water system. Water Air, & Soil Pollution, 161, 157–173. DOI: 10.1007/s11270-005-2993-8.
Devillers, J., Bintein, S., & Domine, D. (1996). Comparison of BCF models based on log P. Chemosphere, 33, 1047–1065. DOI: 10.1016/0045-6535(96)00246-9.
Gramatica, P., Giani, E., & Papa, E. (2007). Statistical external validation and consensus modeling: A QSPR case study for Koc prediction. Journal of Molecular Graphics and Modelling, 25, 755–766. DOI: 10.1016/j.jmgm.2006.06.005.
Hester, R. E., & Harrison, R. M. (1996). Chlorinated organic micropollutants. Cambridge, UK: The Royal Society of Chemistry, Thomas Graham House.
Mackay, D. (1982). Correlation of bioconcentration factors. Environmental Sciience & Technology, 16, 274–278. DOI: 10.1021/es00099a008.
Mackay, D. (2001). Multimedia environmental models. The fugacity approach. Boca Raton, FL, USA: CRC Press.
Mackay, D., & Fraser, A. (2000). Bioaccumulation of persistent organic chemicals: mechanisms and models. Environmental Pollution, 110, 375–391. DOI: 10.1016/s0269-7491(00)00162-7.
Maria, G. (2004). A review of algorithms and trends in kinetic model identification for chemical and biochemical systems. Chemical and Biochemical Engineering Quarterly, 18, 195–222.
Maria, G., & Rippin, D. W. T. (1993). Note concerning two techniques for complex kinetic pathway analysis. Chemical Engineering Science, 48, 3855–3864. DOI: 10.1016/0009-2509(93)80228-i.
Maria, G., & Maria, C. (2006). Evaluation of risk zones over a river pathway, downstream a release point, under seasonal pollutant biodegradability. Chemical and Biochemical Engineering Quarterly, 20, 333–342.
Maria, C., & Maria, G. (2008). Prediction of dispersion and bioaccumulation of chlorinated benzene pollutants in a river section for a low level of discharge. Revista de Chimie, 59, 1122–1131.
Maria, G., & Maria, C. (2009). Bioaccumulation dynamics of a PCB low-level discharge in a riverine pathway downstream the release point. Chemical and Biochemical Engineering Quarterly, 23, 121–134.
MathWorks (2002). Statistics toolbox for use with Matlab. User’s guide (Version 4). Natick, MA, USA: MathWorks, Inc. Retreived July 15, 2012, from http://www.mathworks.com/help/releases/R13sp2/pdfdoc/stats/stats.pdf
McCarty, L. S., Dixon, D. G., Ozburn, G. W., & Smith, A. D. (1992). Toxicokinetic modeling of mixtures of organic chemicals. Environmental Toxicology and Chemistry, 11, 1037–1047. DOI: 10.1002/etc.5620110716.
Merck (2007). ChemDAT Merck database, München, Germany: Merck.
Middleton, G. V. (2000). Data analysis in the Earth sciences using Matlab. New Jersey, NJ, USA: Prentice Hall.
SPAWAR Systems Center (2006). Ex-ORISKANY artificial reef project. San Diego, CA, USA: Space and Naval Warfare Systems Center.
NRCG (2001). Report on hydrological (pollutant dispersion) models. Petten, The Netherlands: Nuclear Research & Consultancy Group.
OECD (1996). OECD guidelines for the testing of chemicals. Bioconcentration: Flow-through fish test. 305. Paris, France.
Papa, E., Dearden, J. C., & Gramatica, P. (2007). Linear QSAR regression models for the prediction of bioconcentration factors by physicochemical properties and structural theoretical molecular descriptors. Chemosphere, 67, 351–358. DOI: 10.1016/j.chemosphere.2006.09.079.
Ramaswami, A., Milford, J. B., & Small, M. J. (2005). Integrated environmental modelling. Pollutant transport, fate, and risk in the environment. Hoboken, NJ, USA: Wiley.
Shiu, W. Y., Wania, F., Hung, H., & Mackay, D. (1997). Temperature dependence of aqueous solubility of selected chlorobenzenes, polychlorinated biphenyls, and dibenzofuran. Journal of Chemical & Engineering Data, 42, 293–297. DOI: 10.1021/je960299u.
Sigma-Aldrich (2010). Safety data sheet. Technical services. Retrieved July 20, 2012, from http://www.sigmaaldrich.com/catalog/AdvancedSearchPage.do
TOXNET (2011). Hazardous substances data bank (HSDB). Retrieved December 10, 2011, from http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
University of Akron (2010). The chemical database. Retrieved July 15, 2012, from 〈http://ull.chemistry.uakron.edu/erd/〉
US EPA (2011). Soil screening guidance: Fact sheet. EPA/540/F-95/041. Washington, DC, USA: US Environmental Protection Agency.
van Leeuwen, C. J., & Vermeire, T. G. (2007). Risk assessment of chemicals: An introduction. Dordrecht, The Netherlands: Springer.
Van Wijk, D., Thompson, R. S., De Rooij, C., Garny, V., Lecloux, A., & Kanne, R. (2004). 1,2-dichlorobenzene marine risk assessment with special reference to the Osparcom region: North Sea. Environmental Monitoring and Assessment, 97, 87–102. DOI: 10.1023/b:emas.0000033043.73470.98.
Verloop, A., Hoogenstraaten, W., & Tipker, J. (1976). Development and application of new steric substituent parameters in drug design. In E. J. Ariens (Ed.), Drug design (pp. 165–207). New York, NY, USA: Academic Press.
Verschueren, K. (2001). Handbook of environmental data on organic chemicals. New York, NY, USA: Wiley.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maria, C., Tociu, C. & Maria, G. Improvement of aquatic pollutant partition coefficient correlations using 1D molecular descriptors — chlorobenzene case study. Chem. Pap. 67, 173–185 (2013). https://doi.org/10.2478/s11696-012-0257-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0257-9