Abstract
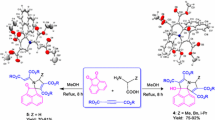
Aminohydroxylation of prochiral divinylcarbinol and subsequent Pd(II)-catalysed oxy-/amidocarbonylation of aminopentenediols is reported. The method was applied to the preparation of useful building blocks for syntheses of cytotoxic jaspines and glycosidase inhibitor DLX-homologues. The key intermediates, tetrahydrofuranolactones (l-arabino-II) and pyrrolidinolactones (l-arabino-IX and l-xylo-IX), were prepared in a short 2-step sequence from divinylcarbinol.
Similar content being viewed by others
References
Abraham, E., Davies, S. G., Roberts, P. M., Russell, A. J., & Thomson, J. E. (2008). Jaspine B (pachastrissamine) and 2-epi-jaspine B: synthesis and structural assignment, Tetrahedron: Asymmetry, 19, 1027–1047. DOI: 10.1016/j.tetasy.2008.04.017.
Angelaud, R., Landais, Y., & Schenk, K. (1997). Asymmetric amino-hydroxylation of dienylsilanes. An efficient route to amino-cyclitols. Tetrahedron Letters, 38, 1407–1410. DOI: 10.1016/s0040-4039(97)00079-8.
Babjak, M., Kapitán, P., & Gracza, T. (2002). The first total synthesis of goniothalesdiol. Tetrahedron Letters, 43, 6983–6985. DOI: 10.1016/s0040-4039(02)01599-x.
Babjak, M., Kapitán, P., & Gracza, T. (2005). Synthesis of (+)-goniothalesdiol and (+)-7-epi-goniothalesdiol. Tetrahedron, 61, 2471–2479. DOI: 10.1016/j.tet.2005.01.004.
Bashyal, B. P., Fleet, G. W. J., Gough, M. J., & Smith, P. W. (1987). Synthesis of the α-Mannosidase inhibitors swainsonine[(1S,2R,8R,8aR)-1,2,8-trihydroxyoctahydroindolizine] and 1,4-dideoxy-1,4-imino-d-mannitol from mannose. Tetrahedron, 43, 3083–3093. DOI: 10.1016/s0040-4020(01) 86850-2.
Bhaket, P., Morris, K., Stauffer, C. S., & Datta, A. (2005). Total synthesis of cytotoxic anhydrophytosphingosine pachastrissamine (jaspine B). Organic Letters, 7, 875–876. DOI: 10.1021/ol0473290.
Canals, D., Mormeneo, D., Fabriàs, G., Llebaria, A., Casas, J., & Delgado, A. (2009). Synthesis and biological properties of Pachastrissamine (jaspine B) and diastereoisomeric jaspines. Bioorganic & Medicinal Chemistry, 17, 235–241. DOI: 10.1016/j.bmc.2008.11.026.
Crimmins, M. T., Ellis, J. M., Emmitte, K. A., Haile, P. A., McDougall, P. J., Parrish, J. D., & Zuccarello, J. L. (2009). Enantioselective total synthesis of Brevetoxin A: Unified strategy for the B, E, G, and J subunits. Chemistry-A European Journal, 15, 9223–9234. DOI: 10.1002/chem.200900776.
Corey, E. J., Noe, M. C., & Guzman-Perez, A. (1995). Kinetic resolution by enantioselective dihydroxylation of secondary allylic 4-methoxybenzoate esters using a mechanistically designed cinchona alkaloid catalyst. Journal of the American Chemical Society, 117, 10817–10824. DOI: 10.1021/ja00149a004.
Dixon, D. J., Ley, S. V., Gracza, T., & Szolcsányi, P. (1999). Total synthesis of the polyenoyltetramic acid mycotoxin erythroskyrine. Journal of the Chemical Society, Perkin Transactions 1, 1999, 839–842. DOI: 10.1039/a809823i.
Enders, D., Terteryan, V., & Paleček, J. (2008). Asymmetric synthesis of jaspine B (pachastrissamine) via an organocatalytic aldol reaction as key step. Synthesis, 2008, 2278–2282. DOI: 10.1055/s-2008-1067142.
Fleet, G. W. J., Nicholas, S. J., Smith, P. W., Evans, S. V., Fellows, L. E., & Nash, R. J. (1985). Potent competitive inhibition of α-galactosidase and α-glucosidase activity by 1,4-dideoxy-1,4-iminopentitols: syntheses of 1,4-dideoxy-1,4-imino-d-lyxitol and both enantiomers of 1,4-dideoxy-1,4-iminoarabinitol. Tetrahedron Letters, 26, 3127–3130. DOI: 10.1016/s0040-4039(00)98636-2.
Gracza, T., Hasenöhrl, T., Stahl, U., & Jäger, V. (1991). Synthesis of 3,5-anhydro-2-deoxy-1,4-glyconolactones by palladium (II)-catalyzed, regioselective oxycarbonylation of C5- and C6-enitols. ω-Homologation of aldoses to produce intermediates for C-glycoside/C-nucleoside synthesis. Synthesis, 1991, 1108–1118 DOI: 10.1055/s-1991-28400.
Gracza, T., & Jäger, V. (1992). Palladium(II)-catalyzed oxycarbonylation of unsaturated polyols: Synthesis of (−)-goniofufurone and assignment of absolute configuration to the natural (+)-enantiomer, a cytotoxic styryllactone. Synlett, 1992, 191–193. DOI: 10.1055/s-1992-21309.
Gracza, T., & Jäger, V. (1994). Synthesis of natural and unnatural enantiomers of goniofufurone and its 7-epimers from Dglucose. Application of palladium(II)-catalyzed oxycarbonylation of unsaturated polyols. Synthesis, 1994, 1359–1368. DOI: 10.1055/s-1994-25694.
Häfele, B., Schröter, D., & Jäger, V. (1986). SynSynthese von erythro-d- und l-4-Pententriolen; selektive Epoxyallylalkohol-hydrolyse unter Retention oder zweifacher Inversion (Enantiomerisierung). Angewandte Chemie, 98, 89–90. DOI: 10.1002/ange.19860980115.
Hümmer, W., Dubois, E., Gracza, T., & Jäger, T. (1997). Halocyclization and palladium(II)-catalyzed amidocarbonylation of unsaturated aminopolypols. Synthesis of 1,4-iminoglycitols as potential glycosidase inhibitors. Synthesis, 1997, 634–642. DOI: 10.1055/s-1997-3183.
Inuki, S., Yoshimitsu, Y., Oishi, S., Fujii, N., & Ohno, H. (2009). Ring-construction/stereoselective functionalization cascade: Total synthesis of pachastrissamine (jaspine B) through palladium-catalyzed bis-cyclization of bromoallenes. Organic Letters, 11, 4478–4481. DOI: 10.1021/ol901904w.
Inuki, S., Yoshimitsu, Y., Oishi, S., Fujii, N., & Ohno, H. (2010). Ring-construction/stereoselective functionalization cascade: Total synthesis of pachastrissamine (jaspine B) through palladium-catalyzed bis-cyclization of propargyl chlorides and carbonates. The Journal of Organic Chemistry, 75, 3831–3842. DOI: 10.1021/jo100544v.
Jäger, V., & Hümmer, W. (1990). CCyclisierung N-geschützter 1-Amino-4-penten-2,3-diole zu lyxo-konfigurierten Desoxyiminozuckern (cis-Dihydroxypyrrolidinen); Synthese potentieller Glycosidase-Inhibitoren. Angewandte Chemie, 102, 1182–1183 DOI: 10.1002/ange.19901021022.
Jäger, V., Gracza, T., Dubois, E., Hasenöhrl, T., Hümmer, W., Kautz, U., Kirschbaum, B., Lieberknecht, A., Remeň, L., Shaw, D., Stahl, U., & Stephan, O. (1997). Pd(II)-catalyzed carbonylation of unsaturated polyols and aminopolyols. In G. Helmchen (Ed.), Proceedings of the Fifth Symposium Organic Synthesis via Organometallics (OSM5), September 26–28, 1996 (pp. 331–360). Braunschweig/Wiesbaden, Germany: Vieweg & Sohn Verlagsgesellschaft.
Karlubíková, O., Babjak, M., & Gracza, T. (2011). Tetrahydropyran synthesis by palladium(II)-catalysed hydroxycarbonylation of hexenols: synthesis of (±)-diospongin A and (+)-civet cat compound. Tetrahedron, 67, 4980–4987. DOI: 10.1016/j.tet.2011.04.045.
Kuroda, I., Musman, M., Ohtani, I. I., Ichiba, T., Tanaka, J., Gravalos, D. G., & Higa, T. (2002). Pachastrissamine, a cytotoxic anhydrophytosphingosine from a marine sponge, Pachastrissa sp. Journal of Natural Products, 65, 1505–1506. DOI: 10.1021/np010659y.
Ledroit, V., Debitus, C., Lavaud, C., & Massiot, G. (2003). Jaspines A and B: two new cytotoxic sphingosine derivatives from the marine sponge Jaspis sp. Tetrahedron Letters, 44, 225–228. DOI: 10.1016/s0040-4039(02)02541-8.
Li, G., Chang, H. T., & Sharpless, K. B. (1996). Catalytic asymmetric aminohydroxylation (AA) of olefins. Angewandte Chemie International Edition, 35, 451–454. DOI: 10.1002/anie.199604511.
Llaveria, J., Díaz, Y., Matheu, M. I., & Castillón, S. (2011). Enantioselective synthesis of jaspine B (pachastrissamine) and its C-2 and/or C-3 epimers. European Journal of Organic Chemistry, 2011, 1514–1519. DOI: 10.1002/ejoc.201001477.
Nicolaou, K. C., Rodríguez, R. M., Mitchell, H. J., & van Delft, F. L. (1998). Stereocontrolled synthesis of the everninomicin A1B(A)C ring framework. Angewandte Chemie International Edition, 37, 1874–1876. DOI: 10.1002/(SICI)1521-3773(19980803)37:13/14〈1874::AID-ANIE1874〉3.0.CO;2-K.
Passiniemi, M., & Koskinen, A. M. P. (2011). Asymmetric synthesis of Pachastrissamine (Jaspine B) and its diastereomers via η 3-allylpalladium intermediates. Organic & Biomolecular Chemistry, 99, 1774–1783. DOI: 10.1039/c0ob00643b.
Reddy, K. L., & Sharpless, K. B. (1998). From styrenes to enantiopure α-arylglycines in two steps. Journal of the American Chemical Society, 120, 1207–1217. DOI: 10.1021/ja9728177.
Romero, A., & Wong, C. H. (2000). Chemo-enzymatic total synthesis of 3-epiaustraline, australine, and 7-epialexine. The Journal of Organic Chemistry, 65, 8264–8268. DOI: 10.1021/jo000933d.
Salma, Y., Ballereau, S., Maaliki, C., Ladeira, S., Andrieu-Abadie, N., & Génisson, Y. (2010). Flexible and enantioselective access to jaspine B and biologically active chainmodified analogues thereof. Organic & Biomolecular Chemistry, 8, 3227–3243. DOI: 10.1039/c004218h.
Schreiber, S. L., Schreiber, T. S., & Smith, D. B. (1987). Reactions that proceed with a combination of enantiotopic group and diastereotopic face selectivity can deliver products with very high enantiomeric excess: experimental support of a mathematical model. Journal of the American Chemical Society, 109, 1525–1529. DOI: 10.1021/ja00239a036.
Singh, S., & Guiry, P. J. (2009). Microwave-assisted synthesis of substituted tetrahydropyrans catalyzed by ZrCl4 and its application in the asymmetric synthesis of exo- and endobrevicomin. The Journal of Organic Chemistry, 74, 5758–5761. DOI: 10.1021/jo901019u.
Srinivas Rao, G., & Venkateswara Rao, B. (2011). A common strategy for the stereoselective synthesis of anhydrophytosphingosine pachastrissamine (jaspine B) and N,O,O,O-tetra-acetyl d-lyxo-phytosphingosine. Tetrahedron Letters, 52, 6076–6079. DOI: 10.1016/j.tetlet.2011.08.170.
Tao, B., Schlingloff, G., & Sharpless, K. B. (1998). Reversal of regioselection in the asymmetric aminohydroxylation of cinnamates. Tetrahedron Letters, 39, 2507–2510. DOI: 10.1016/s0040-4039(98)00350-5.
Urano, H., Enomoto, M., & Kuwahara, S. (2010). Enantioselective syntheses of pachastrissamine and jaspine A via hydroxylactonization of a chiral epoxy ester. Bioscience, Biotechnology, and Biochemistry, 74, 152–157. DOI: 10.1271/bbb.90670.
Vichare, P., & Chattopadhyay, A. (2010). Nitrolaldol reaction of (R)-2,3-cyclohexylideneglyceraldehyde: a simple and stereoselective synthesis of the cytotoxic Pachastrissamine (Jaspine B). Tetrahedron: Asymmetry, 21, 1983–1987. DOI: 10.1016/j.tetasy.2010.06.024.
Yoshimitsu, Y., Inuki, S., Oishi, S., Fujii, N., & Ohno, H. (2010). Stereoselective divergent synthesis of four diastereomers of pachastrissamine (jaspine B). The Journal of Organic Chemistry, 75, 3843–3846. DOI: 10.1021/jo1005284.
Yoshimitsu, Y., Oishi, S., Miyagaki, J., Inuki, S., Ohno, H., & Fujii, N. (2011). Pachastrissamine (jaspine B) and its stereoisomers inhibit sphingosine kinases and atypical protein kinase C. Bioorganic & Medicinal Chemistry, 19, 5402–5408. DOI: 10.1016/j.bmc.2011.07.061.
Wang, H., Matsuhashi, H., Doan, B. D., Goodman, S. N., Ouyang, X., & Clark, W. M., Jr. (2009). Large-scale synthesis of SB-462795, a cathepsin K inhibitor: the RCM-based approaches. Tetrahedron, 65, 6291–6303. DOI: 10.1016/j.tet.2009.06.022.
Winkler, D. A., & Holan, G. (1989). Design and potential anti-HIV agents. 1. Mannosidase inhibitors. Journal of Medicinal Chemistry, 32, 2084–2089. DOI: 10.1021/jm00129a011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Štefan Toma on the occasion of his 75th birthday
Rights and permissions
About this article
Cite this article
Caletková, O., Ďurišová, D., Prónayová, N. et al. Aminohydroxylation of divinylcarbinol and its application to the synthesis of bicyclic hydroxypyrrolidine and aminotetrahydrofuran building blocks. Chem. Pap. 67, 66–75 (2013). https://doi.org/10.2478/s11696-012-0224-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0224-5