Abstract
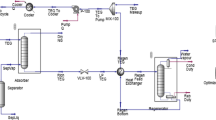
Using the simulation program CHEMCAD, performance characteristics, design optimization, and costs of an absorption/stripping system used to purify 100 kg h−1 of biogas in a biogas power plant were investigated. Potential absorbents used in the chemical absorption process were the following aqueous solutions: pure diglycolamine, diglycolamine/piperazine, and diglycolamine/methyldiethanolamine/piperazine. Mixtures for agricultural biogas purification to below 1 vol. % of CO2 and 4 × 10−4 mass % of H2S were determined via a simulation in the above mentioned program. The chosen mixtures were then entered into an absorption/desorption system and simulations for each unit were provided by CHEMCAD. From the simulation results, the design parameters were calculated and entered into each unit’s “cost estimation” section in the aforementioned program to estimate the purchase costs of the apparatuses. Taking into account the installation, maintenance, as well as other additional costs, the actual machine purchase costs were multiplied by the Lang factor. Costs of additional streams were also calculated by multiplying the ten-year utility losses by their respective cost factors. From these calculations, the absorbent mixture, allowing biogas production at the lowest estimated costs for ten years, was found.
Similar content being viewed by others
References
Ahmad, Z. (2006). Principles of corrosion engineering and corrosion control (pp. 125). Oxford, UK: Elsevier.
AISC (1989). AISC Manual of steel construction: Allowable stress design (9th ed.). Chicago, IL, USA: American Institute of Steel Construction.
Arnold, K., & Stewart, M. (1989). Surface production operations: Design of gas handling systems and facilities (2nd ed.). Houston, TX, USA: Gulf.
Baasel, W. D. (1990). Preliminary chemical engineering plant design (2nd ed., pp. 272). Melbourne, Australia: Elsevier.
Barreau, A., Blanchon le Bouhelec, E., Habchi Tounsi, K. N., Mougin, P., & Lecomte, F. (2006). Absorption of H2S and CO2 in alkanolamine aqueous solution: Experimental data and modeling with the electrolyte-NRTL model. Oil & Gas Science and Technology — Revue de l’IFP, 61, 345–361. DOI: 10.2516/ogst:2006038a.
Bell, K. J., & Mueller, A. C. (1984). Wolverine engineering data book II. Shawnee, OK, USA: Wolverine Tube Inc.
Bishnoi, S., & Rochelle, G. T. (2002). Thermodynamics of piperazine/methyldiethanolamine/water/carbon dioxide. Industrial & Engineering Chemistry Research, 41, 604–612. DOI: 10.1021/ie0103106.
Brownell, L. E., & Young, E. H. (1959). Process equipment design: Vessel design (pp. 172). New York, NY, USA: Wiley.
Bullin, J. A., Polasek, J. C., & Donnelly, S. T. (1990). The use of MDEA and mixtures of amines for bulk CO2 removal. In Proceedings of the 69th Gas Processors Association Annual Convention, March 12–13, 1990 (pp. 135–139). Tulsa, OK, USA: Gas Processors Association.
Chen, C. C., & Evans, L. B. (1986). A local composition model for the excess Gibbs energy of aqueous electrolyte systems. AICHE Journal, 32, 444–454. DOI: 10.1002/aic.690320311.
Chung, T. S. (2009). Expert assessments of retrofitting coalfired power plants with carbon dioxide capture technologies (pp. 26–29). Masters project, Nicolas School of the Environment of Duke University, Durham, NC, USA.
Cullinane, J. T., & Rochelle, G. T. (2003). Properties of concentrated aqueous potassium carbonate/piperazine for CO2 capture. In The 2nd Annual Conference on Carbon Sequestration, May 5–8, 2003. Alexandria, VA, USA.
Dang, H., & Rochelle, G. T. (2001). CO2 absorption rate and solubility in monoethanolamine/piperazine/water. In The 1st National Conference on Carbon Sequestration, May 14–17, 2001. Washington, DC, USA.
Dubois, L., & Thomas, D. (2009). CO2 absorption into aqueous solutions of monoethanolamine, methyldiethanolamine, piperazine and their blends. Chemical Engineering & Technology, 32, 710–718. DOI: 10.1002/ceat.200800545.
Green, D. W., & Perry, R. H. (2008). Perry’s Chemical engineers’ handbook (8th ed.). Portland, ME: Tata McGraw-Hill.
Kohl, A. L., & Nielsen, R. B. (1997). Gas purification (5th ed.). Houston, TX, USA: Gulf.
Lang, H. J. (1948). Simplified approach to preliminary cost estimates. Chemical Engineering, NY, 55(6), 112–113.
Lindeburg, M. R. (2006). Mechanical engineering reference manual for the PE exam (12th ed., pp. 18–8). Belmont, CA, USA: Professional Publications.
Luyben, W. L. (2011). Principles and case studies of simultaneous design. Hoboken, NJ, USA: Wiley.
Manning, F. S., & Thompson, R. E. (1991). Oilfield processing of petroleum: Natural gas (pp. 116). Tulsa, OK, USA: PennWell.
Oyenekan, B. A., & Rochelle, G. T. (2009). Rate modeling of CO2 stripping from potassium carbonate promoted by piperazine. International Journal of Greenhouse Gas Control, 3, 121–132. DOI: 10.1016/j.ijggc.2008.06.010.
Pacheco, M. A., Kaganoi, S., & Rochelle, G. T. (2000). CO2 absorption into aqueous mixtures of diglycolamine and methyldiethanolamine. Chemical Engineering Science, 55, 5125–5140. DOI: 10.1016/s0009-2509(00)00104-4.
Pitts, D. R., & Sissom, L. E. (1998). Schaum’s outline of theory and problems of heat transfer (2nd ed., pp. 54). New York, NY, USA: McGraw-Hill.
Polasek, J. C., & Bullin, J. A. (1994). Selecting amines for sweetening units. In Proceedings of the GPA Regional Meeting “Process Considerations in Selecting Amine”, September, 1994. Tulsa, OK, USA: Gas Processors Association.
Rousseau, R. W. (1987). Handbook of separation process technology (pp. 293). New York, NY, USA: Wiley.
Salkuyeh, Y. K., & Mofarahi, M. (2011). Comparison of MEA and DGA performance for CO2 capture under different operational conditions. International Journal of Energy Research, 36, 259–268. DOI: 10.1002/er.1812.
Savage, D. W., & Funk, E. W. (1981). Selective absorption of H2S and CO2 into aqueous solutions of methyldiethanolamine. In 90th AIChE National Meeting, April 5–9, 1981. Houston, TX, USA.
Seader, J. D., & Henley, E. J. (2006). Separation process principles (2nd ed.). Danvers, MA, USA: Wiley.
Seider W. D., Seader, J. D., & Lewin, R. L. (2003). Product and process design principles: Synthesis, analysis and evaluation (2nd ed.). New York, NY, USA: Wiley.
Sinnott, R. K., & Towler, G. (2009). Chemical engineering design (5th ed.). Oxford, UK: Elsevier.
Sohbi, B., Meakaff, M., Emtir, M., & Elgarni, M. (2007). The using of mixing amines in an industrial gas sweetening plant. World Academy of Science, Engineering and Technology, 31(1), 301–305.
Souders, M., & Brown, G. G. (1934). Design of fractionating columns I. Entrainment and capacity. Industrial & Engineering Chemistry, 26, 98–103. DOI: 10.1021/ie50289a025.
Thakore, S. B., & Bhatt, B. I. (2007). Introduction to process engineering and design. New Delhi, India: Tata McGraw-Hill.
Zare Aliabad, H., & Mirzaei, S. (2009). Removal of CO2 and H2S using aqueous alkanolamine solutions. World Academy of Science, Engineering and Technology, 49(1), 194–203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gawel, R.A. Design simulations for a biogas purification process using aqueous amine solutions. Chem. Pap. 66, 1010–1018 (2012). https://doi.org/10.2478/s11696-012-0211-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0211-x