Abstract
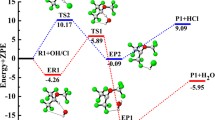
Reaction mechanism of 1,1,1-trifluorotrichloroethane (CF3CCl3) and sulphur trioxide (SO3) in the presence of mercury salts (Hg2SO4 and HgSO4) was studied applying the density functional theory (DFT) at the UB3LYP/6-31+G(d,p) level. It was found that this reaction occurs in the free radical chain path as follows: mercury(I) sulphate free radical is generated by heat, causing CF3CCl3 to produce the CF3CCl2 free radical which reacts with SO3 leading to the formation of CF3CCl2OSO2 decomposing into CF3COCl and SO2Cl. The SO2Cl free radical triggers CF3CCl3 to regenerate CF3CCl2 which recycles the free radical growth reaction. This elementary reaction has the highest energy barrier and it is therefore the rate control step of the whole reaction path. Experiment data can confirm the existence of the mercury(I) salt free radical and the free radical initiation stage. So, mercury salts play the role of initiators not that of catalysts. The results agree well with our hypothesis.
Similar content being viewed by others
References
Anello, L. G., Eibeck, R. E., & Robinson, M. A. (1982). U.S. Patent No. 4340548. Washington, D.C., USA: U.S. Patent and Trademark Office.
Benning, A. F, & Park, J. D. (1946). U.S. Patent No. 2396076. Washington, D.C., USA: U.S. Patent and Trademark Office.
Christian, G. D. (2003). Analytical chemistry (6th ed.). New York, NY, USA: Wiley.
Farnell, L., Pople, J. A., & Radom, L. (1983). Structural predictions for open-shell systems: a comparative assessment of ab initio procedures. The Journal of Physical Chemistry, 87, 79–82. DOI: 10.1021/j100224a019.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Jr., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, N. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2010). Gaussian09, Revision B.01 [computer software]. Wallingford, CT, USA: Gaussian, Inc.
House, J. E., & House, K. A. (2010). Descriptive inorganic chemistry (2nd ed.). Burlington, MA, USA: Academic Press.
Lei, Y., Sun, Q., Chen, Z., & Shen, J. (2009). Theoretical calculations on the thermodynamics for the synthesis reactions of polyoxymethylene dimethyl ethers. Acta Chimica Sinica, 67, 767–772.
Liu, J., Zheng, C. G.,& Qiu, J. R. (2007). Studies on quantum chemistry calculation method of mercury reactions in combustion flue gas. Kung Cheng Je Wu Li Hsueh Pao/Journal of Engineering and Thermophysics, 28, 519–521.
Marangoni, L., Guglielmo, G., & Conte, L. (1980). Preparation of CF3COCl: investigation of operating parameters. Journal of Fluorine Chemistry, 16, 572. DOI: 10.1016/s0022-1139(00)84084-1.
March, J. (1992). Advanced organic chemistry: Reactions, mechanisms, and structure (4th ed.). New York, NY, USA: Wiley-Interscience.
Paucksch, H., Massonne, J., Bohm, H., & Fernschild, G. (1973). U.S. Patent No. 3725475. Washington, D.C., USA: U.S. Patent and Trademark Office.
Qiu, Y. X, Fang, H., Zhang, Y., & Wang, S. G. (2004). Theoretical studies on the structure of Hg(I)-hydroxidic compounds. Acta Chimica Sinica, 62, 556–560.
Rogers, D. W. (2011). Concise physical chemistry. New York, NY, USA: Wiley.
Ruudorufu, U., Fuerushirudo, G., & Hirushu, U. (1981). JP Patent No. 56501649. Tokyo, Japan: Japan Patent Office.
Schlegel, H. B., & Sosa, C. (1988). Ab initio molecular orbital calculations on F+H2 → HF+H and OH+H2 → H2O+H using unrestricted Moller-Plesset perturbation theory with spin projection. Chemical Physics Letters, 145, 329–333. DOI: 10.1016/0009-2614(88)80016-2.
Wang, H., Yang, H., Di, G., Wen, Z., Ran, X., Shi, Q., Luo, R., & Yang, Y. (2001). DFT kinetic study of the pyrolysis mechanism of toluene used for carbon matrix. Acta Chimica Sinica, 59, 17–21.
Wei, T. J., & Zhang, J. W. (1990). Assay of chloroacetyl chloride. Chinese Journal of Pharmaceuticals, 21, 25–26.
Yoshida, T., Kanetani, T., & Misaki, S. (1985). JP Patent No. 60237040. Tokyo, Japan: Japan Patent Office.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, ZZ., Chen, ZR., Yin, H. et al. Mechanistic insights into the reaction of CF3CCl3 with SO3: Theory and experiment. Chem. Pap. 66, 1059–1064 (2012). https://doi.org/10.2478/s11696-012-0205-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0205-8