Abstract
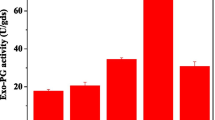
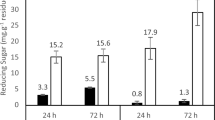
Geotrichum candidum CCY 16-1-29 (teleomorph Galactomyces geotrichum) is able to grow and produce polygalacturonase of remarkable activities on pectin or grape pomace as a sole carbon source. The highest activities of extracellular enzymes were found on the third and the seventh day of cultivation. After extraction and precipitation, polygalacturonases produced in these cultivation periods were characterized. Production of multiple forms of polygalacturonase was observed in both cultivation periods. Two major forms, polygalacturonase with random action pattern (endo-PGase, EC 3.2.1.15) and oligogalacturonate hydrolase (exoPGase, exopolygalacturonase preferring oligogalacturonides as substrates), as well as numerous minor forms were detected by IEF-PAGE using the print technique detection. EndoPGase was identified by mass spectrometry. The major forms have similar isoelectric points (below pH 6.0) and pH optima (4.6 and 4.8, respectively). pH optimum of 4.6 was associated with exoPGase and that of 4.8 with endoPGase. Both enzymes were stable after freeze-drying and storage at 4°C. EndoPGase had molecular mass of about 29 kDa (36 kDa by SDS-PAGE) as determined by gel filtration, temperature optimum of about 45°C and it was stable only below 35°C. Molecular mass of exoPGase was about 50 kDa, its temperature optimum was about 60°C, and it was stable to 60°C. Optimal substrate for exoPGase was a pentamer, for endoPGase it was a pectate. Values of K m for optimal substrate reached the values of 11.4 × 10−5 M for for exoPGase and 6.6 × 10−5 M for endoPGase. Pectin methylesterase as another pectolytic enzyme was also identified by mass spectrometry.
Similar content being viewed by others
References
Barash, I., & Eyal, Z. (1969). Properties of a polygalacturonases produced by Geotrichum candidum. Phytopathology, 60, 27–30. DOI: 10.1094/phyto-60-27.
Barash, I., Zilberman, E., & Marcus, L. (1984). Purification of Geotrichum candidum endopolygalacturonase from culture and from host tissue by affinity chromatography on crosslinked polypectate. Physiological Plant Pathology, 25, 161–169. DOI: 10.1016/0048-4059(84)90054-7.
Birren, B. W., Lander, E. S., Galagan, J. E., Nusbaum, C., Devon, K., Ma, L. J., Jaffe, D. B., Butler, J., Alvarez, P., Gnerre, S., Grabherr, M., Kleber, M., Mauceli, E. W., Brockman, W., MacCallum, I. A., Young, S. K., LaButti, K., De-Caprio, D., Crawford, M., Koehrsen, M., Engels, R., Montgomery, P., Pearson, M., Howarth, C., Larson, L., White, J., Yandava, C., Kodira, C. D., Guigo, R., Borodovsky, M., Zeng, Q., O’Leary, S., Alvarado, L., Pandelova, I., & Ciuffetti, L. (2007). Genome sequence of Pyrenophora triticirepentis. In EMBL/GenBank/DDBJ databases.
Blanco, P., Sieiro, C., & Villa, T. G. (1999). Production of pectic enzymes in yeasts. FEMS Microbiology Letters, 175, 1–9. DOI: 10.1016/s0378-1097(99)00090-7.
Ellwood, S. R., Liu, Z. H., Syme, R. A., Lai, Z. B., Hane, J. K., Keiper, F., Moffat, C. S., Oliver, R. P., & Friesen, T. L. (2010). A first genome assembly of the barley fungal pathogen Pyrenophora teres f. teres.Genome Biology, 11, R109.1–R109.14. DOI: 10.1186/gb-2010-11-11-r109.
Flodrová, D., Dzúrová, M., Lišková, D., Ait Mohand, F., Mislovičová, D., Malovíková, A., Voburka, Z., Omelková, J., & Stratilová, E. (2007). Pectate hydrolases of parsley (Petroselinum crispum) roots. Zeitschrift für Naturforschung, 62c, 382–388.
Guillotin, S. (2005). Studies on the intra- and intermolecular distributions of substituents in commercial pectins. PhD. thesis, Wageningen University, The Netherlands.
Hang, Y. D., & Woodams, E. E. (1992). Production and characterization of polygalacturonase from Geotrichum candidum. World Journal of Microbiology and Biotechnology, 8, 480–482. DOI: 10.1007/bf01201944.
Heinrichová, K. (1983). Preparation of oligogalacturonic acids by enzymatic hydrolysis. Biologia, Bratislava, 38, 335–342.
Iguchi, K., Hirano, H., Kishida, M., Kawasaki, H., & Sakai, T. (1997). Cloning of a protopectinase gene of Trichosporon penicillatum and its expression in Saccharomyces cerevisiae. Microbiology, 143, 1657–1664. DOI: 10.1099/00221287-143- 5-1657.
Jayani, R. S., Saxena, S., & Gupta, R. (2005). Microbial pectinolytic enzymes: A review. Process Biochemistry, 40, 2931–2944. DOI: 10.1016/j.procbio.2005.03.026.
Jensen, O. N., Wilm, M., Shevchenko, A., & Mann, M. (1999). Sample preparation methods for mass spectrometric peptide mapping directly from 2-DE gels. In A. J. Link (Ed.), Methods in molecular biology: 2-D proteome analysis protocols (Chapter 52, pp. 513-530). Totowa, NJ, USA: Humana Press. DOI: 10.1385/1-59259-584-7:513.
Kohn, R., & Furda, I. (1967). Calcium ion activity in solutions of calcium pectinate. Collection of Czechoslovak Chemical Communications, 32, 1925–1937.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Markovič, O., Mislovičová, D., Biely, P., & Heinrichová, K. (1992). Chromogenic substrate for endo-polygalacturonase detection in gels. Journal of Chromatography A, 603, 243–246. DOI:10.1016/0021-9673(92)85367-3.
Nakamura, M., Iwai, H., & Arai, K. (2002). Cloning and characterization of polygalacturonase gene Ap2pg1 from Geotrichum candidum citrus race Ap2 pathogenic to apple fruit. Journal of General Plant Pathology, 68, 333–337. DOI: 10.1007/pl00013099.
Nakamura, M., Iwai, H., & Arai, K. (2003). Polygalacturonase S31PG1 from Geotrichum candidum citrus race S31 expressed in Schizosaccharomyces pombe versus S31PG2 regarding soft rot on lemon fruit. Journal of General Plant Pathology, 69, 283–291. DOI: 10.1007/s10327-003-0048-9.
Nakamura, M., Suprapta, D. N., Iwai, H., & Arai, K. (2001). Comparison of endo-polygalacturonase activities of citrus and non-citrus races of Geotrichum candidum, and cloning and expression of the corresponding genes. Molecular Plant Pathology, 2, 265–274. DOI: 10.1046/j.1464- 6722.2001.00075.x.
Palanivelu, P. (2006). Polygalacturonases: Active site analyses and mechanism of action. Indian Journal of Biotechnology, 5, 148–162.
Radola, B. J. (1980). Ultrathin-layer isoelectric focusing in 50–100 μm polyacrylamide gels on silanized glass plates or polyester films. Electrophoresis, 1, 43–56. DOI: 10.1002/elps.1150010109.
Rexová-Benková, Ľ. (1970). Separation of oligogalacturonic acids by dextran gel chromatography. Chemické Zvesti, 24, 59–62.
Rexová-Benková, Ľ., & Markovič, O. (1976). Pectic enzymes. Advances in Carbohydrate Chemistry and Biochemistry, 33, 323–385. DOI: 10.1016/s0065-2318(08)60285-1.
Rouxel, T., Grandaubert, J., Hane, J. K., Hoede, C., van de Wouw, A. P., Couloux, A., Dominguez, V., Anthouard, V., Bally, P., Bourras, S., Cozijnsen, A. J., Ciuffetti, L. M., Degrave, A., Dilmaghani, A., Duret, L., Fudal, I., Goodwin, S. B., Gout, L., Glaser, N., Linglin, J., Kema, G. H. J., Lapalu, N., Lawrence, C. B., May, K., Meyer, M., Ollivier, B., Poulain, J., Schoch, C. L., Simon, A., Spatafora, J. W., Stachowiak, A., Turgeon, B. G., Tyler, B. M., Vincent, D., Weissenbach, J., Amselem, J., Quesneville, H., Oliver, R. P., Wincker, P., Balesdent, M. H., & Howlett, B. J. (2011). Effector diversification within compartments of the Leptosphaeria maculans genome affected by Repeat- Induced Point mutations. Nature Communications, 2, 202. DOI: 10.1038/ncomms1189.
Schols, H. A., Visser, R. G. F., & Voragen, A. G. J. (2009). Pectins and pectinases. Wagenigen, The Netherlands: Wageningen Academic Publishers.
Somogyi, M. (1952). Notes on sugar determination. The Journal of Biological Chemistry, 195, 19–23.
Stratilová, E., Dzúrová, M., Breierová, E., & Omelková, J. (2006). Production and biochemical characterization of polygalacturonases produced by Aureobasidium pullulans from forest soil. Annals of Microbiology, 56, 35–40. DOI: 10.1007/bf03174967.
Stratilová, E., Dzúrová, M., Malovíková, A., & Omelková, J. (2005). Oligogalacturonate hydrolase from carrot roots. Zeitschrift für Naturforschung, 60c, 899–905.
Visser, J., & Voragen, A. G. J. (1996). Progress in Biotechnology (Vol. 14, Pectin and pectinases). Amsterdam, The Netherlands: Elsevier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Illková, K., Zemková, Z., Flodrová, D. et al. Production of Geotrichum candidum polygalacturonases via solid state fermentation on grape pomace. Chem. Pap. 66, 852–860 (2012). https://doi.org/10.2478/s11696-012-0189-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0189-4