Abstract
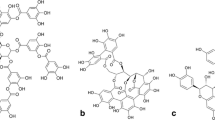
Tannic acid is commonly employed as the main component in culture media for the selection of tannase-producing strains. In biotechnological processes it is the favorite substrate used to induce the tannase enzyme in both solid and submerged culture for microbial and/or enzymatic production of gallic acid. However, the results found in literature are inconsistent notwithstanding the strict control of all parameters that rule the bioprocesses. The present work, for the first time, reveals the importance of differences in the quality and chemical profile of tannic acid from different suppliers and their influence on the fungal and enzymatic hydrolytic pattern obtained when it is used as a substrate. A degree of hydrolysis between 64.7 % and 100 % has been determined in different tannic acid samples. The specific growth rate of 0.712 h−1, 0.792 h−1, 0.477 h−1, 0.536 h−1 for Jalmek®, Faga Lab®, Division Food®, and Riedel de Häen®, respectively, was obtained at the concentration of 80 g L−1 of each of the tannic acids.
Similar content being viewed by others
References
Aguilar, C. N., Augur, C., Favela-Torres, E., & Viniegra-González, G. (2001). Induction and repression patterns of fungal tannase in solid-state and submerged cultures. Process Biochemistry, 36, 565–570. DOI: 10.1016/s0032-9592(00)00251-x.
Aguilar, C. N., & Gutiérrez-Sánchez, G. (2001). Review: Sources, properties, applications and potential uses of tannin acyl hydrolase. Food Science and Technology International, 7, 373–382. DOI: 10.1106/69m3-b30k-cf7q-rj5g.
Aguilera-Carbo, A., Augur, C., Prado-Barragan, L. A., Favela-Torres, E., & Aguilar, C. N. (2008). Microbial production of ellagic acid and biodegradation of ellagitannins. Applied Microbiology and Biotechnology, 78, 189–199. DOI: 10.1007/s00253-007-1276-2.
Banerjee, D., Mahapatra, S., & Pati, B. R. (2007). Gallic acid production by submerged fermentation of Aspergillus aculeatus DBF9. Research Journal of Microbiology, 2, 462–468. DOI: 10.3923/jm.2007.462.468.
Banerjee, D., & Pati, B. R. (2007). Optimization of tannase production by Aureobasidium pullulans DBS66. Journal of Microbiology and Biotechnology, 17, 985–992.
Belmares, R., Contreras-Esquivel, J. C., Rodríguez-Herrera, R., Ramírez Coronel, A., & Aguilar, C. N. (2004). Microbial production of tannase: an enzyme with potential use in food industry. LWT-Food Science and Technology, 37, 857–864. DOI: 10.1016/j.lwt.2004.04.002.
Bhat, T. K., Singh, B., & Sharma, O. P. (1998). Microbial degradation of tannins — A current perspective. Biodegradation, 9, 343–357. DOI: 10.1023/a:1008397506963.
Bollen, W. B., & Lu, K. C. (1969). Douglas-fir bark tannin decomposition in two forest soils. Portland, OR, USA: Pacific Northwest Forest and Range Experiment station.
Clarke, I. D., Rogers, J. S., Sievers, A. F., & Hopp, H. (1949). Tannin content and other characteristics of native sumac in relation to its value as a commercial source of tannin. Technical Bulletin of the United States Department of Agriculture, 986, 1–76.
Cruz-Hernández, M., Contreras, J. C., Lima, N., Teixeira, J., & Aguilar, C. (2009). Production of Aspergillus niger GH1 tannase using solid-state fermentation. Journal of Pure and Applied Microbiology, 3, 21–26.
Das Mohapatra, P. K., Mondal, K. C., & Pati, B. R. (2006). Production of tannase through submerged fermentation of tannin-containing plant extracts by Bacillus licheniformis KBR6. Polish Journal of Microbiology, 55, 297–301.
Goldstein, J. L., & Swain, T. (1965). The inhibition of enzymes by tannins. Phytochemistry, 4, 185–192. DOI: 10.1016/s0031- 9422(00)86162-2.
Haslam, E. (1989). Plant polyphenols: vegetable tannins revisited. New York, NY, USA: Cambridge University Press.
Khanbabaee, K., & van Ree, T. (2001). Tannins: Classification and definition. Natural Product Reports, 18, 641–649. DOI: 10.1039/b101061l.
Lekha, P. K., & Lonsane, B. K. (1997). Production and application of tannin acyl hydrolase: State of the art. Advances in Applied Microbiology, 44, 215–260. DOI: 10.1016/s0065-2164(08)70463-5.
Li, M., Yao, K., He, Q., & Jia, D. (2006). Biodegradation of gallotannins and ellagitannins. Journal Basic Microbiology, 46, 68–84. DOI: 10.1002/jobm.200510600.
Li, W. W., Li, X. D., & Zeng, K. M. (2009). Aerobic biodegradation kinetics of tannic acid in activated sludge system. Biochemical Engineering Journal, 43, 142–148. DOI: 10.1016/j.bej.2008.09.010.
Mata-Gómez, M., Rodríguez, L. V., Ramos, E. L., Renovato, J., Cruz-Hernández, M. A., Rodríguez, R., Contreras, J., & Aguilar, C. N. (2009). A novel tannase from the xerophilic fungus Aspergillus niger GH1. Journal of Microbiology and Biotechnology, 19, 987–996. DOI: 10.4014/jmb.1009.09041.
Mueller-Harvey, I. (2001). Analysis of hydrolysable tannins. Animal Feed Science and Technology, 91, 3–20. DOI: 10.1016/s0377-8401(01)00227-9.
Salminen, J. P., Ossipov, V., Loponen, J., Haukioja, E., & Pihlaja, K. (1999). Characterization of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography—mass spectrometry. Journal of Chromatography A, 864, 283–291. DOI: 10.1016/s0021-9673(99)01036-5.
Schofield, P., Mbugua, D. M., & Pell, A. N. (2001). Analysis of condensed tannins: a review. Animal Feed Science and Technology, 91, 21–40. DOI: 10.1016/s0377-8401(01)00228-0.
Sharma, S., Bhat, T. K., & Dawra, R. K. (2000). A spectrophotometric method for assay of tannase using rhodanine. Analytical Biochemistry, 279, 85–89. DOI: 10.1006/abio.1999.4405.
Swain, T., & Bate-Smith, E. C. (1962). Flavonoid compounds. In H. S. Mason, & A. M. Florkin (Eds.), Comparative biochemistry (pp. 755–809). New York, NY, USA: Academic Press.
Van Diepeningen, A. D., Debets, A. J. M., Varga, J., Van Der Gaag, M., Swart, K., & Hoekstra, R. F. (2004). Efficient degradation of tannic acid by black Aspergillus species. Mycological Reserch, 108, 919–925. DOI: 10.1017/s0953756204000747.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chávez-González, M.L., Contreras-Esquivel, J.C., Prado-Barragán, L.A. et al. Microbial and enzymatic hydrolysis of tannic acid: influence of substrate chemical quality. Chem. Pap. 66, 171–177 (2012). https://doi.org/10.2478/s11696-011-0112-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-011-0112-4