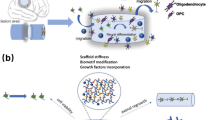
Abstract
The intrinsically multi-factorial pathological trend of spinal cord injury is probably the most important reason behind the absence of efficient therapeutic strategies. Therefore, recent studies suggest the use of new tools combining the delivery of both cells and drugs. Systems which are able to perform multiple controlled delivery of different therapeutic agents have gained particularly strong interest. Hence, in order to avoid trial and error approaches, several studies were performed following the classic chemical engineering multiscale approach: tuning microchemistry to manipulate macro properties in order to satisfy specific medical needs as injectability, low stress on target tissues, ability to retain liquids, capability of carrying living cells, and possibility to control the delivery of drugs. In this framework we focused on injectable agarose-carbomer based hydrogels applying he results of our studies performed in the past two years: in vitro biocompatibility, physical chemical studies, drug delivery transport phenomena investigation, and in vivo biocompatibility in uninjured Brainbow mice.
Similar content being viewed by others
References
Annabi, N., Nichol, J. W., Zhong, X., Ji, C., Koshy, S., Khademhosseini, A., & Dehghani, F. (2010). Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Engineering: Part B, 16, 371–383. DOI: 10.1089/ten.teb.2009.0639.
Atala, A., Lanza, R., Thomson, J. A., & Nerem, R. M. (2008). Principles of regenerative medicine. Burlington, MA, USA: Elsevier.
Bacaj, T., Tevlin, M., Lu, Y., & Shaham, S. (2008). Glia are essential for sensory organ function in C. elegans. Science, 322, 744–747. DOI: 10.1126/science.1163074.
Baumann, M. D., Kang, C. E., Stanwick, J. C., Wang, Y., Kim, H., Lapitsky, Y., & Shoichet, M. S. (2009). An injectable drug delivery platform for sustained combination therapy. Journal of Controlled Release, 138, 205–213. DOI: 10.1016/j.jconrel.2009.05.009.
Baumann, M. D., Kang, C. E., Tator, C. H., & Shoichet, M. S. (2010). Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials, 31, 7631–7639. DOI: 10.1016/j.biomaterials.2010.07.004.
Bjugstad, K. B., Lampe, K., Kern, D. S., & Mahoney, M. (2010). Biocompatibility of poly(ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. Journal of Biomedical Materials Research Part A, 95A, 79–91. DOI: 10.1002/jbm.a.32809.
Bradbury, E. J., & Carter, L. M. (2011). Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Research Bulletin, 84, 306–316. DOI: 10.1016/j.brainresbull.2010.06.015.
Brännvall, K., Bergman, K., Wallenquist, U., Svahn, S., Bowden, T., Hilborn, J., & Forsberg-Nilsson, K. (2007). Enhanced neuronal differentiation in a three-dimensional collagenhyaluronan matrix. Journal of Neuroscience Research, 85, 2138–2146. DOI: 10.1002/jnr.21358.
Calegari, F., Coco, S., Taverna, E., Bassetti, M., Verderio, C., Corradi, N., Matteoli, M., & Rosa, P. (1999). A regulated secretory pathway in cultured hippocampal astrocytes. The Journal of Biological Chemistry, 274, 22539–22547. DOI: 10.1074/jbc.274.32.22539.
Cao, K., Huang, L., Liu, J., An, H., Shu, Y., & Han, Z. (2010). Inhibitory effects of high-dose methylprednisolone on bacterial translocation from gut and endotoxin re lease following acute spinal cord injury-induced paraplegia in rats. Neural Regeneration Research, 5, 456–460. DOI: 10.3969/j.issn.1673-5374.2010.06.009.
Casalini, T., Salvalaglio, M., Perale, G., Masi, M., & Cavallotti, C. (2011). Diffusion and aggregation of sodium fluorescein in aqueous solutions. Journal of Physical Chemistry B, in press. DOI: 10.1021/jp207459k.
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., Littman, D. R., Dustin, M. L., & Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8, 752–758. DOI: 10.1038/nn1472.
de Jong, S. J., van Eerdenbrugh, B., van Nostrum, C. F., Kettenes-van de Bosch, J. J., & Hennink, W. E. (2001). Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: degradation and protein release behavior. Journal of Controlled Release, 71, 261–275. DOI: 10.1016/s0168-3659(01)00228-0.
Dumitriu, S. (2002). Polymeric biomaterials. New York, NY: Marcel Dekker.
European Commision (2009). Commission regulation (EC) No 668/2009 of 24 July 2009. Official Journal of the European Union, L 194, 7–10.
Fawcett, J.W., & Asher, R. A. (1999). The glial scar and central nervous system repair. Brain Research Bulletin, 49, 377–391. DOI: 10.1016/s0361-9230(99)00072-6.
Flemming, R. G., Murphy, C. J., Abrams, G. A., Goodman, S. L., & Nealey, P. F. (1999). Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials, 20, 573–588. DOI: 10.1016/s0142-9612(98)00209-9.
Flory, P. J. (1953). Principles of polymer chemistry. New York, NY, USA Cornell Univeristy Press.
Fournier, E., Passirani, C., Montero-Menei, C. N., & Benoit, J. P. (2003). Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials, 24, 3311–3331. DOI: 10.1016/s0142-9612(03)00161-3.
Hejčl, A., Lesny, P., Přadný, M., Šedý, J., Zámečník, J., Jendelová, P., Michálek, J., & Syková, E. (2009). Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 6: 3D hydrogels with positive and negative surface charges and polyelectrolyte complexes in spinal cord injury repair. Journal of Material Science: Materials in Medicine, 20, 1571–1577. DOI: 10.1007/s10856-009-3714-4.
Hejčl, A., Šedý, J., Kapcalová, M., Toro, D. A., Amemori, T., Lesny, P., Likavčanová-Mašínová, K., Krumbholcová, E., Přándý, M., Michálek, J., Burian, M., Hájek, M., Jendelová, P., & Syková, E. (2010). HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells and Development, 19, 1535–1546. DOI: 10.1089/scd.2009.0378.
Horner, P. J., & Gage, F. H. (2000). Regenerating the damaged central nervous system. Nature, 407, 963–970. DOI: 10.1038/35039559.
Huglin, M. B., Rehab, M. M. A. M., & Zakaria, M. B. (1986). Thermodynamic interactions in copolymeric hydrogels. Macromolecules, 19, 2986–2991. DOI: 10.1021/ma0016 6a019.
Kim, Y. T., Caldwell, J. M., & Bellamkonda, R. V. (2009). Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury. Biomaterials, 30, 2582–2590. DOI: 10.1016/j.biomaterials.2008.12.077.
Kubinová, Š., & Syková, E. (2010). Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine, 5, 99–108. DOI: 10.2217/nnm.09.93.
Kwon, B. K., Sekhon, L. H., & Fehlings, M. G. (2010). Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine, 35, S263–S270. DOI: 10.1097/brs.0b013e3181f3286d.
Langer, R. (2009). Perspectives and challenges in tissue engineering and regenerative medicine. Advanced Materials, 21, 3235–3236. DOI: 10.1002/adma.200902589.
Lanza, R. P., Langer, R., & Vacanti, J. (2000). Principles of tissue engineering. Burlington, MA, USA: Elsevier.
Leung, B. K., Biran, R., Underwood, C. J., & Tresco, P. A. (2008). Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials, 29, 3289–3297. DOI: 10.1016/j.biomaterials.2008.03.045.
Livet, J., Weissman, T. A., Kang, H., Draft, R.W., Lu, J., Bennis, R. A., Sanes, J. R., & Lichtman, J.W. (2007). Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature, 450, 56–62. DOI: 10.1038/nature06293.
Luo, Y., & Shoichet, M. S. (2004). A photolabile hydrogel for guided three-dimensional cell growth and migration. Nature Materials, 3, 249–253. DOI: 10.1038/nmat1092.
McDonald, J. W., Gottlieb, D. I., & Choi, D. W. (2000). Reply to “What is a functional recovery after spinal cord injury?”. Nature Medicine, 6, 358. DOI: 10.1038/74759.
Nakamatsu, J., Torres, F. G., Troncoso, O. P., Yuan, M. L., & Boccaccini, A. R. (2006). Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules, 7, 3345–3355. DOI: 10.1021/bm0605311.
Nisbet, D. R., Crompton, K. E., Horne, M. K., Finkelstein, D. I., & Forsythe, J. S. (2008). Neural tissue engineering of the CNS using hydrogels. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 87B, 251–263. DOI: 10.1002/jbm.b.31000.
Novikova, L. N., Mosahebi, A., Wiberg, M., Terenghi, G., Kellerth, J. O., & Novikov, L. N. (2006). Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. Journal of Biomedical Materials Research Part A, 77A, 242–252. DOI: 10.1002/jbm.A.30603.
Perale, G., Giordano, C., Bianco, F., Rossi, F., Tunesi, M., Daniele, F., Crivelli, F., Matteoli, M., & Masi, M. (2011a). Hydrogel for cell housing in the brain and in the spinal cord. International Journal of Artificial Organs, 34, 295–303. DOI: 10.5301/ijao.2011.6488.
Perale, G., Rossi, F., Santoro, M., Marchetti, P., Mele, A., Castiglione, F., Raffa, E., & Masi, M. (2011b). Drug release from hydrogel: A new understanding of transport phenomena. Journal of Biomedical Nanotechnology, 7, 476–481. DOI: 10.1166/jbn.2011.1302.
Perale, G., Rossi, F., Sundstrom, E., Bacchiega, S., Masi, M., Forloni, G., & Veglianese, P. (2011c). Hydrogels in spinal cord injury repair strategies. ACS Chemical Neuroscience, 2, 336–345. DOI: 10.1021/cn200030w.
Perale, G., Veglianese, P., Rossi, F., Peviani, M., Santoro, M., Llupi, D., Micotti, E., Forloni, G., & Masi, M. (2011d). In situ agar-carbomer hydrogel polycondensation: A chemical approach to regenerative medicine. Materials Letters, 65, 1688–1692. DOI: 10.1016/j.matlet.2011.02.036.
Prang, P., Müller, R., Eljaouhari, A., Heckmann, K., Kunz, W., Weber, T., Faber, C., Vroemen, M., Bogdahn, U., & Weidner, N. (2006). The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials, 27, 3560–3569. DOI: 10.1016/j.biomaterials.2006.01.053.
Rossi, F., Casalini, T., Santoro, M., Mele, A., & Perale, G. (2011a). Methylprednisolone release from agar-Carbomerbased hydrogel: a promising tool for local drug delivery. Chemical Papers, 65, 903–908. DOI: 10.2478/s11696-011-0059-5.
Rossi, F., Chatzistavrou, X., Perale, G., & Boccaccini, A. R. (2012). Synthesis and degradation of agar-carbomer based hydrogels for tissue engineering appliactions. Journal of Applied Polymer Science, 123, 398–408. DOI: 10.1002/app.34488.
Rossi, F., Perale, G., & Masi, M. (2010). Biological buffered saline solution as solvent in agar-carbomer hydrogel synthesis. Chemical Papers, 64, 573–578. DOI: 10.2478/s11696-010-0052-4.
Rossi, F., Perale, G., Storti, G., & Masi, M. (2011b). A library of tunable agarose carbomer-based hydrogels for tissue engineering applications: The role of cross-linkers. Journal of Applied Polymer Science, in press. DOI: 10.1002/app.34731.
Sakurada, K., McDonald, F. M., & Shimada, F. (2008). Regenerative medicine and stem cell based drug discovery. Angewandte Chemie International Edition, 47, 5718–5738. DOI: 10.1002/anie.200700724.
Santoro, M., Marchetti, P., Rossi, F., Perale, G., Castiglione, F., Mele, A., & Masi, M. (2011). Smart approach to evaluate drug diffusivity in injectable agar-carbomer hydrogels for drug delivery. Journal of Physical Chemistry B, 115, 2503–2510. DOI: 10.1021/jp1111394.
Shoichet, M. S. (2010). Polymer scaffolds for biomaterials applications. Macromolecules, 43, 581–591. DOI: 10.1021/ma901 530r.
Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., & Peppas, N. A. (2009). Hydrogels in regenerative medicine. Advanced Materials, 21, 3307–3329. DOI: 10.1002/adma.200802106.
Stella, V. J., Lee, H. K., & Thompson, D. O. (1995). The effect of SBE4-β-CD on i.v. methylprednisolone pharmacokinetics in rats: Comparison to a co-solvent solution and two water-soluble prodrugs. International Journal of Pharmaceutics, 120, 189–195. DOI: 10.1016/0378-5173(94)00404-S.
Steward, O., Schauwecker, P. E., Guth, L., Zhang, Z. Y., Fujiki, M., Inman, D., Wrathall, J., Kempermann, G., Gage, F. H., Saatman, K. E., Raghupathi, R., & McIntosh, T. (1999). Genetic approaches to neurotrauma research: Opportunities and potential pitfalls of murine models. Experimental Neurology, 157, 19–42. DOI: 10.1006/exnr.1999.7040.
Tan, H. P., Chu, C. R., Payne, K. A., & Marra, K. G. (2009). Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials, 30, 2499–2506. DOI: 10.1016/j.biomaterials.2008.12.080.
van de Manakker, F., Vermonden, T., el Morabit, N., van Nostrum, C. F., & Hennink, W. E. (2008). Rheological behavior of self-assembling PEG-β-cyclodextrin/PEGcholesterol hydrogels. Langmuir, 24, 12559–12567. DOI: 10.1021/la8023748.
van den Berg, M. E. L., Castellote, J. M., de Pedro-Cuesta, J., & Mahillo-Fernandez, I. (2010a). Survival after spinal cord injury: A systematic review. Journal of Neurotrauma, 27, 1517–1528. DOI: 10.1089/neu.2009.1138.
van den Berg, M. E. L., Castellote, J. M., Mahillo-Fernandez, I., & de Pedro-Cuesta, J. (2010b). Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology, 34, 184–192. DOI: 10.1159/000279335.
Varghese, O. P., Sun, W., Hilborn, J., & Ossipov, D. A. (2009). In situ cross-linkable high molecular weight hyaluronanbisphosphonate conjugate for localized delivery and cellspecific targeting: A hydrogel linked prodrug approach. Journal of the American Chemical Society, 131, 8781–8784. DOI: 10.1021/ja902857b.
Willerth, S. M., & Sakiyama-Elbert, S. E. (2007). Approaches to neural tissue engineering using scaffolds for drug delivery. Advanced Drug Delivery Reviews, 59, 325–338. DOI: 10.1016/j.addr.2007.03.014.
Woerly, S., Pinet, E., de Robertis, L., Van Diep, D., & Bousmina, M. (2001). Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGelTM). Biomaterials, 22, 1095–1111. DOI: 10.1016/s0142-9612(00)00354-9.
Zhong, Y., & Bellamkonda, R. V. (2008). Biomaterials for the central nervous system. Journal of the Royal Society Interface, 5, 957–975. DOI: 10.1098/rsif.2008.0071.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perale, G., Rossi, F., Veglianese, P. et al. Chemical engineering approach to regenerative medicine. Chem. Pap. 66, 108–119 (2012). https://doi.org/10.2478/s11696-011-0111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-011-0111-5